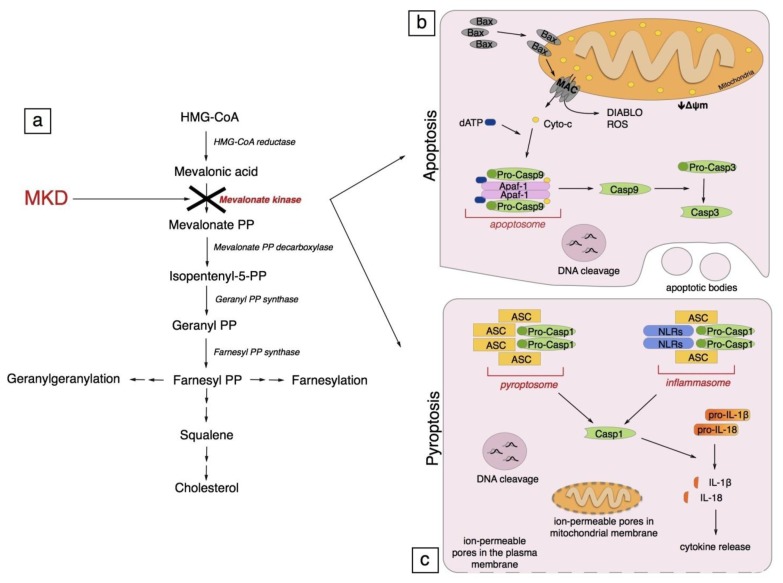
Figure 1.
Mevalonate pathway and programmed cell death. (a) Mevalonate Kinase Deficiency (MKD) is characterized by a decrease of Mevalonate Kinase (MK, in red) residual activity. MK is the second enzyme of the mevalonate pathway. The programmed cell death occurring in MKD is linked to both apoptosis and pyroptosis pathways; (b) Intrinsic apoptosis pathway: BAX (Bcl-2-associated X protein) is activated and then, after oligomerization, it forms a channel into the mitochondria external membrane known as MAC (mitochondrial apoptosis-induced channel). MAC is important for the release of: cytochrome c, DIABLO (a second mitochondria-derived activator of caspase), ROS (reactive oxygen species), and for the dissipation of the mitochondrial transmembrane potential (Δψm). Cytochrome c binds to Apaf-1 (apoptotic protease-activating factor 1), forming the apoptosome that activates caspase-9 in an ATP-dependent manner. Active caspase-9 cleaves and activates effector caspase-3. Active caspase-3 cleaves target proteins that induce the cell death characterized by a DNA cleavage and the development of the membrane-enclosed apoptotic bodies; (c) Pyroptosis is a caspase-1 dependent programmed cell death. Caspase-1 can be activated by pyroptosoma and by inflammasome. Pyroptosoma is composed of oligomerized ASC (apoptosis-associated speck-like protein containing a CARD) dimers; inflammasome is composed of NLRs (nucleotide-binding oligomerization-domain protein-like receptors) and ASC, and both of them activate caspase-1. Active caspase-1 induces: maturation of pro-IL-1β and pro-IL-18 into, respectively, IL-1β and IL-18; DNA cleavage, and the formation of ion-permeable pores in the plasma membrane and in mitochondrial membrane.