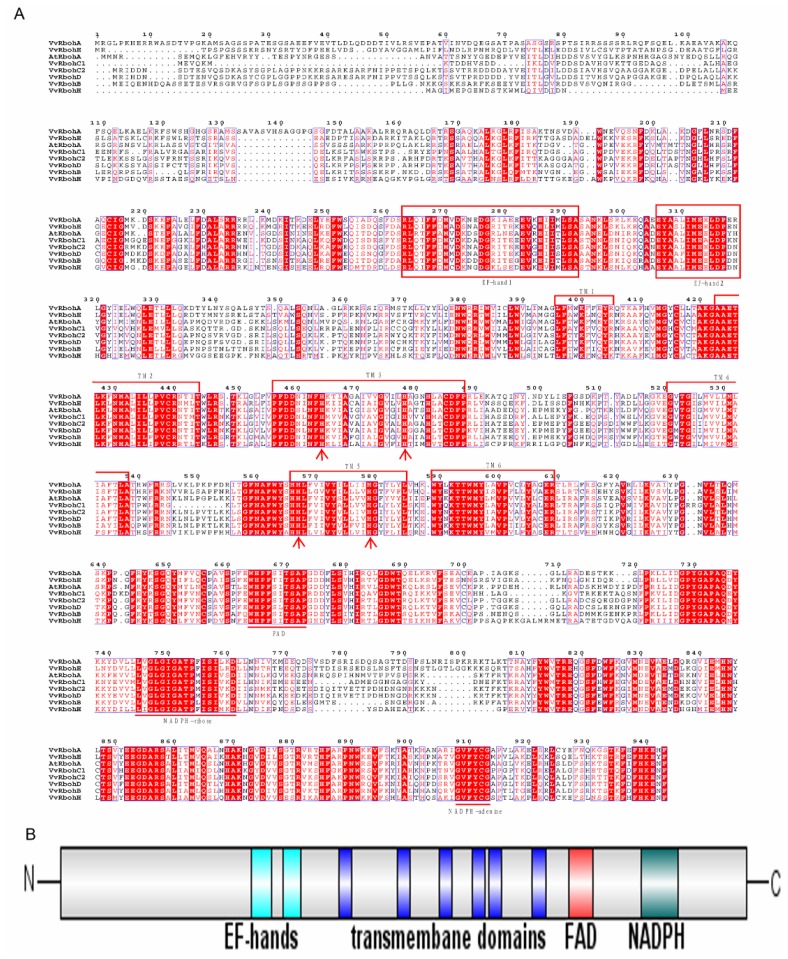
Figure 1.
Protein alignment and domain structure of AtRbohA and the seven predicted grape respiratory burst oxidase homolog (Rboh) proteins. (A) The red shading indicates residues that are identical and the lighter shading represents positions with a lower level of conservation. EF-hand domains are indicated by the boxes. Conserved binding sites for flavin adenine dinucleotide (FAD), NADPH-ribose and NADPH-adenine are indicated with a straight line below the alignment. Histidine residues involved in heme binding are indicated by arrows. Putative transmembrane domains (TMs) are indicated by brackets above the alignment; and (B) Schematic representation of the grape Rboh proteins with their respective functional domains, showing that grape Rboh proteins are similar to Arabidopsis AtRbohA.