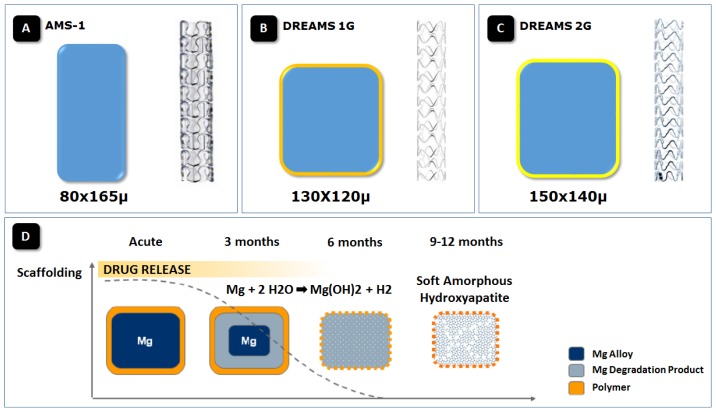
Figure 1.
(A) Schematic cross-sectional profile of magnesium scaffolds struts of (A) uncoated, non-eluting, AMS-1 with 80 × 165 μ; (B) DREAMS 1st Generation (DREAMS 1G) with 130 × 120 μ struts and (C) DREAMS 2nd generation (2G) with 150 × 140 μ struts. The poly(lactide-co-glycolide)-coating with paclitaxel elution of the DREAMS 1G scaffold is indicated by the thin light orange layer. The PLA-coating with sirolimus elution of the DREAMS 2G scaffold is indicated by the thin dark orange layer; and (D) Schematic representation of the resorption process in the drug-eluting absorbable magnesium scaffold. The release of the anti-proliferative drug occurs within the first 3 months after device implantation. Hydrolysis of the scaffold affects the radial strength of the scaffold, resulting in a gradual resorption of the device into a soft amorphous hydroxyapatite at 9 months follow-up. AMS-1, first-generation bare absorbable metal scaffold; DREAMS, Drug-Eluting Absorbable Metal Scaffold.