Significance
Many diseases result from genetic mutations that cause protein misfolding. Medical treatments often address the symptoms, but do not correct the underlying etiology. This study illustrates proof of principle that a disease caused by a misfolded cell surface receptor can be corrected with a pharmacoperone, a unique class of target-specific drugs that assist protein folding.
Keywords: protein trafficking, protein misrouting, intracellular trafficking
Abstract
Mutations in receptors, ion channels, and enzymes are frequently recognized by the cellular quality control system as misfolded and retained in the endoplasmic reticulum (ER) or otherwise misrouted. Retention results in loss of function at the normal site of biological activity and disease. Pharmacoperones are target-specific small molecules that diffuse into cells and serve as folding templates that enable mutant proteins to pass the criteria of the quality control system and route to their physiologic site of action. Pharmacoperones of the gonadotropin releasing hormone receptor (GnRHR) have efficacy in cell culture systems, and their cellular and biochemical mechanisms of action are known. Here, we show the efficacy of a pharmacoperone drug in a small animal model, a knock-in mouse, expressing a mutant GnRHR. This recessive mutation (GnRHR E90K) causes hypogonadotropic hypogonadism (failed puberty associated with low or apulsatile luteinizing hormone) in both humans and in the mouse model described. We find that pulsatile pharmacoperone therapy restores E90K from ER retention to the plasma membrane, concurrently with responsiveness to the endogenous natural ligand, gonadotropin releasing hormone, and an agonist that is specific for the mutant. Spermatogenesis, proteins associated with steroid transport and steroidogenesis, and androgen levels were restored in mutant male mice following pharmacoperone therapy. These results show the efficacy of pharmacoperone therapy in vivo by using physiological, molecular, genetic, endocrine and biochemical markers and optimization of pulsatile administration. We expect that this newly appreciated approach of protein rescue will benefit other disorders sharing pathologies based on misrouting of misfolded protein mutants.
Missense mutations can result in protein misfolding, an event that leads to retention in the endoplasmic reticulum (ER) and degradation by the cellular quality control system (QCS). As a consequence, loss-of-function diseases result (1, 2). G protein-coupled receptors (GPCRs) that are sensitive to these misfolding mutations have been identified in patients with a wide range of diseases (3) Drugs used to alleviate symptoms of these diseases do not correct the root problem, restoration of plasma membrane (PM) expression and function of the misfolded GPCR. In cell cultures, pharmacoperone drugs diffuse into cells and refold ER-retained mutants into a conformation that is acceptable to the QCS, enabling correct routing to the PM and restoration of function. The efficacy of this class of drugs for treating disease caused by misfolded proteins has yet to be established in vivo.
The gonadotropin releasing hormone (GnRH) receptor (GnRHR) is a GPCR expressed in pituitary gonadotropes. Its PM localization enables cells to respond to extracellular GnRH by production and release of the gonadotropins, luteinizing hormone (LH), and follicle stimulating hormone (FSH). These hormones enter the peripheral circulation and regulate gonadal growth, steroidogenesis, and gamete maturation. Many mutants of the GnRHR become misrouted (4, 5) and cannot respond to GnRH, resulting in low serum gonadotropin levels and loss of gonadal function, a disease termed hypogonadotropic hypogonadism (HH) in humans (6).
Our previous studies in cell cultures demonstrated the efficacy of GnRHR-specific pharmacoperones for rescuing misfolded GnRHR mutants from ER retention, leading to PM localization and normal signaling activity (4, 5). Here, we report a mouse model designed to test the therapeutic utility of pharmacoperones in vivo. These mice harbor the mutation GnRHR E90K, which results in HH in humans and in this animal model. The GnRHR E90K protein is misrouted because the substitution of a positively charged K90 breaks the E90-K121 salt bridge. This bridge, between transmembrane segments 1 and 2, creates a structural relation required for acceptability of the receptor to the cellular QCS (7). A pharmacoperone specific to GnRHR was able to restore testis function in Gnrhr E90K mutant mice. Importantly, once routed to the PM, the mutant GPCR functioned normally and the disease symptoms were attenuated. This study demonstrates the in vivo efficacy of pharmacoperone therapy for a disease caused by protein misfolding.
Results
Mutation GnRHR E90K Produces a Mouse with Hypogonadotropic Hypogonadism.
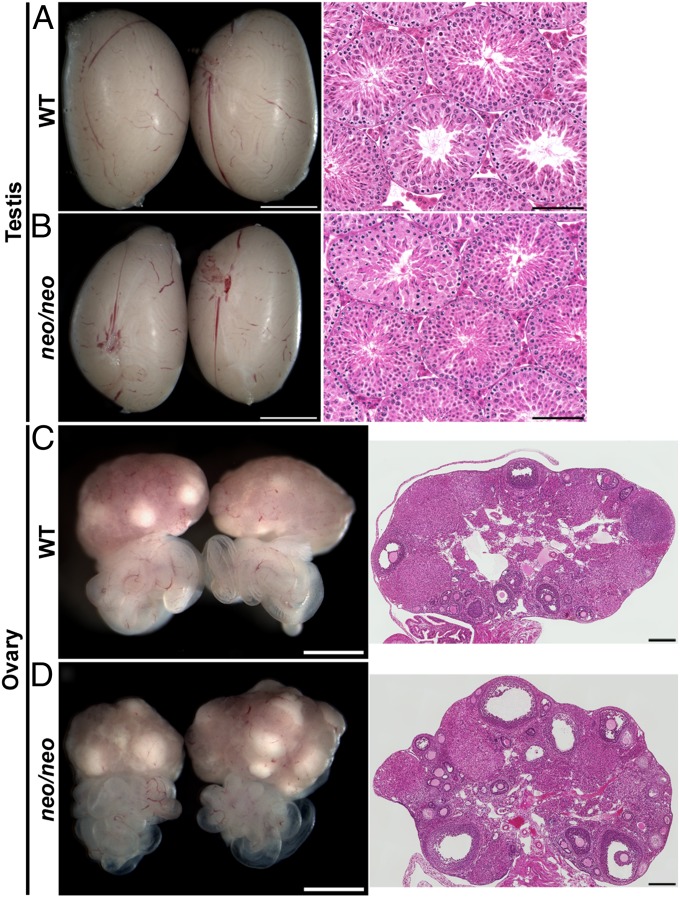
A single base-pair substitution that produces GnRHR E90K was introduced into exon 1 of the mouse Gnrhr locus by homologous recombination in mouse embryonic stem (ES) cells. The gene targeting strategy left a loxP-flanked neomycin resistance gene expression cassette (neo) in intron 1 in reverse orientation to the direction of Gnrhr transcription. Previously, we removed neo and characterized the E90K phenotype. E90K/E90K males have slightly smaller testes compared with controls but are fertile. E90K/E90K females generate antral follicles but do not ovulate (8). Interestingly, when neo was left in the locus, the HH phenotype was more severe, making it a better mouse model for the pharmacoperone trials reported here. As a control, we examined the wild-type (WT) littermates, both male and female mice homozygous for neo alone were fertile and exhibited normal testis and ovary histology (Fig. 1). Thus, neo alone is not sufficient to induce HH. The observation that E90Kneo exhibits a more severe HH phenotype than E90K alone suggests that neo reduces transcription of Gnrhr. The combination of reduced transcription and E90K-induced protein misfolding produces the more severe HH phenotype in E90Kneo mice. E90Kneo/E90Kneo males and females exhibit a strong HH phenotype, but one that is less severe than the previously isolated L117P allele, which is equivalent to a null allele (9). These results indicate that the E90Kneo allele is a strong hypomorph.
Fig. 1.
The presence of the neo cassette alone does not produce hypogonadism. Mice were generated that harbor the neo expression cassette in the context of WT Gnrhr to determine the effect of neo alone on gonadal function. Testes or ovaries from mice of the indicated genotypes were collected at 90 d of age, imaged with a stereomicroscope, and then processed for H&E staining. Both male and female mice homozygous for neo were fertile and exhibited gross morphology and histology comparable to that of WT. The stereomicroscope image is shown in Left and the H&E-stained section is shown on Right. (Scale bars: A–D Left, 2 mm; A and B, Right, 0.1 mm; C and D, Right, 0.2 mm.)
Males were chosen for pharmacoperone infusion experiments; however, E90Kneo homozygous females exhibited HH as well. E90Kneo heterozygous females were fertile, but homozygous mutants were infertile. At the gross and microscopic levels, the ovaries of E90Kneo heterozygous females were indistinguishable from WT. The ovaries of homozygous mutants were small and lacked follicular development past the secondary follicle stage (Fig. S1).
A Catheter Enables Pharmacoperone Infusion to the Pituitary Gonadotropes.
A catheter was inserted in the left carotid of 60-d-old male E90Kneo/E90Kneo mice and fitted to a jacket system that enabled the mouse free-range with access to food and water ad libitum. The catheter positioning was confirmed at autopsy.
Pharmacoperone IN3 ((2S)-2-[5-[2-(2-azabicyclo[2.2.2]oct-2-yl)-1,1-dimethyl-2-oxo-ethyl]-2-(3,5-dimethylphenyl)-1H-indol-3-yl]-N-(2-pyridin-4-ylethyl)propan-1-amine) (Merck and Company) is an antagonist of the GnRHR. We hypothesized that LH release in response to a GnRHR agonist would be attenuated after IN3 infusion. We used this assumption to determine whether IN3 was being delivered to pituitary gonadotropes in our infusion model. To this end, we evaluated the concentration of serum LH 60 min after injection of 10 µg of Buserelin (a GnRHR agonist, administered s.c. in 50 µL of saline). Before being catheterized, animals responded to 10 µg of Buserelin with a serum LH of 8.2 ± 1.1 ng/mL LH, n =3. They were then infused with saline-heparin (SH) for 6 d to allow recovery of pituitary LH and adaptation. IN3 (5 µg/mL, 25 µL/h) was then infused for 6 h (the last hour of which was concurrent with s.c. Buserelin administration) and selected to allow endogenous LH (released in response to endogenous GnRH) to be cleared. Ten micrograms of Buserelin was administered as described. Circulating LH levels were <0.2 ng/mL, n = 3. These data show suppression of LH after IN3 infusion and support the notion that IN3 is efficiently reaching pituitary gonadotropes in our infusion model.
Pulsatile Infusion of Pharmacoperone IN3 Rescues Testicular Weight.
The search for pharmacoperones of misfolded mutants of the GnRHR has relied on repurposing peptidomimetics that were initially identified as receptor antagonists. Because they are small and hydrophobic, these molecules diffuse into cells. Because they are antagonists, it is necessary to wash out the molecule after treatment to allow endogenous ligand binding to the refolded receptor. For this reason, we first optimized the pattern of administration so as to promote its action as a pharmacoperone and minimize antagonistic action.
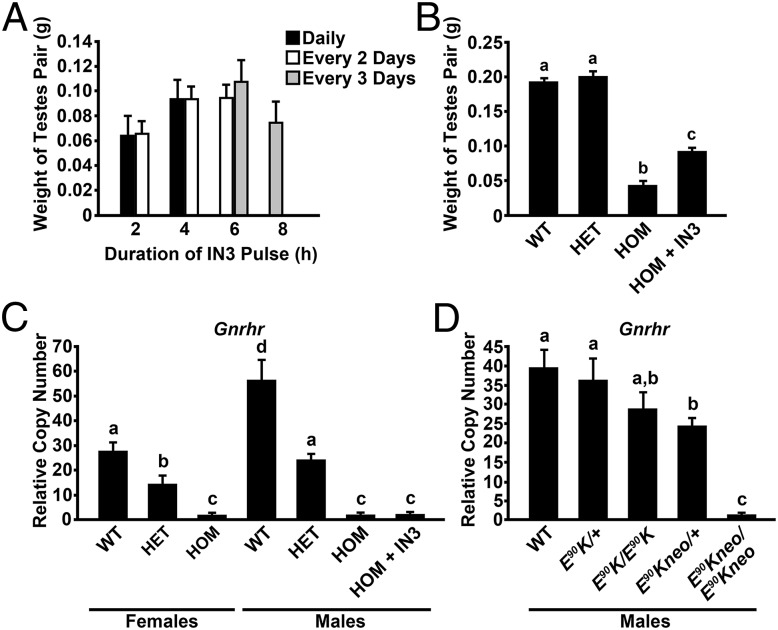
Male E90Kneo/E90Kneo animals displayed severely decreased gonadal size (0.04 g ± 0.005 SEM per 2 testes; n = 19) compared with WT animals (0.19 g ± 0.004 per 2 testes; n = 26) or heterozygotes (0.20 g ± 0.007 per 2 testes; n = 16) at 60 d of age. Male animals were infused at a constant rate of 25 µL/h with SH or 5 µg/mL pharmacoperone IN3 in SH. We varied the frequency (pulses per day) and pulse duration (hours) of pharmacoperone administration in the model animal to identify conditions that allowed rescue of the mutant, followed by washout of the rescue agent. A selection of conditions is shown (Fig. 2A). The optimum drug infusion conditions produced, in 30 d, animals with a mean testis weight (0.11 g per 2 testes; n ≥ 3). When 60-d-old animals were continuously infused with saline or with IN3 for 30 d additionally, testicular weights (sum of left and right) were 0.07 g ± 0.01 and 0.07 g ± 0.01 per 2 testes, respectively.
Fig. 2.
(A) Testicular weight was determined in 60-d-old animals that were infused for 30 d with pharmacoperone IN3 each, 1, 2, or 3 d with pulse duration of 2, 4, 6, or 8 h (n ≥ 3 per group). When animals were continuously infused with saline or with IN3 for 30 d, testicular weights (sum of left and right) were 0.07 ± 0.01 and 0.07 ± 0.01 g, respectively (n ≥ 3 per group). (B) Comparison of testicular weights from WT, E90Kneo heterozygotes (HET), E90Kneo homozygotes (HOM), and E90Kneo homozygotes following a 30-d IN3 regimen (HOM+IN3). (C) Gnrhr qPCR: animals containing the neo cassette. Real-time PCR was performed by using total RNA extracted from the pituitary. Data were analyzed by the ΔΔCT method and reported as copy number relative to that of Gapdh. Females. Gnrhr expression was reduced in E90Kneo heterozygous females compared with WT. Gnrhr expression was further reduced in E90Kneo homozygotes, which exhibit the HH phenotype. For males, Gnrhr expression was reduced in E90Kneo heterozygous males compared with WT. Gnrhr expression was further reduced in E90Kneo homozygotes, which exhibit the HH phenotype. Pharmacoperone IN3 treatment did not affect Gnrhr mRNA levels. For females, for WT, E90Kneo/+ and E90Kneo/E90Kneo groups, n = 8, 3, and 6, respectively. For males, for WT, E90Kneo/+, E90Kneo/E90Kneo, and IN3 groups, n = 5, 11, 8, and 4, respectively. (D) Gnrhr qPCR: Comparison between males harboring only the E90K mutation and those in which the neo cassette is retained. Real-time PCR was performed and data were analyzed as described in A. Gnrhr expression was lower in E90Kneo heterozygotes than in WT or either E90K genotype. E90Kneo homozygous males exhibited the lowest Gnrhr mRNA expression of any genotype. For WT, E90K/+, E90K/E90K, E90Kneo/+, and E90Kneo/E90Kneo groups, n = 6, 8, 7, 13, and 9, respectively. Significant differences (P < 0.05) are denoted by the lowercase letters above each bar (a, b, c). Equivalent means have the same letter; different letters indicate statistically significant differences. Error bars show SEM.
We next compared testis weights for WT, E90Kneo heterozygotes, E90Kneo homozygotes, and E90Kneo homozygotes after IN3 infusion. Testis weights were similar for WT and E90Kneo heterozygotes. E90Kneo homozygotes, which exhibit HH, showed a significant reduction in testis weight. IN3 treatment for 30 d increased testis weight in the homozygotes, but not to WT levels (Fig. 2B).
Serum LH Response to an Agonist of Mutant E90K Is Promoted by Pulsatile IN3 Infusion but Not a Constant Infusion of IN3.
We compared the LH response of pulsatile IN3-treated mutant animals (5 µg/mL IN3 for 6 h every third day for 30 d) to the response from animals infused with either a constant infusion of IN3 or constant SH for 30 d. After constant SH or IN3 or pulsatile IN3 treatments, animals were infused for 1 h with 100 µg/mL Asp2-[GnRH], an agonist of mutant E90K that is not recognized by the WT receptor (10). Serum LH was then measured by radioimmunoassay. Animals receiving constant infusion of IN3 or SH showed <0.2 ng/mL serum LH (n = 3). Animals receiving pulses of IN3 showed serum levels of 9.7 ± 4.2 ng/mL (SEM) LH (n = 3) following Asp2-GnRH. These data suggest that pulsatile IN3 rescued the E90K mutant, whereas constant IN3 was unable to do so. For this reason, the pulsatile IN3 regime was used for all further studies.
Pharmacoperone IN3 Does Not Influence Transcription Levels of the Gnrhr Gene.
Gnrhr mRNA transcript levels in both males and females were reduced in E90Kneo heterozygotes compared with WT and further reduced in homozygous mutants, consistent with the view that neo reduces transcription of the Gnrhr gene. Pharmacoperone IN3 did not significantly influence transcript levels of Gnrhr, consonant with a role of the pharmacoperone solely in protein folding (Fig. 2C).
To test whether the reduction in Gnrhr mRNA expression was due to neo and not E90K, we compared Gnrhr mRNA expression in pituitaries from male E90K mice lacking neo versus E90Kneo by real-time PCR. The results of this assay showed no effect of E90K alone but a significant reduction in Gnrhr mRNA levels in E90Kneo animals (Fig. 2D). These data are consistent with the idea that the reduction in Gnrhr mRNA expression is due to the presence of neo and not due to the E90K mutation. Neo may negatively affect mRNA expression by suppressing transcription, causing aberrant splicing or destabilizing Gnrhr transcripts.
Pharmacoperone Infusion Improves Testis Function.
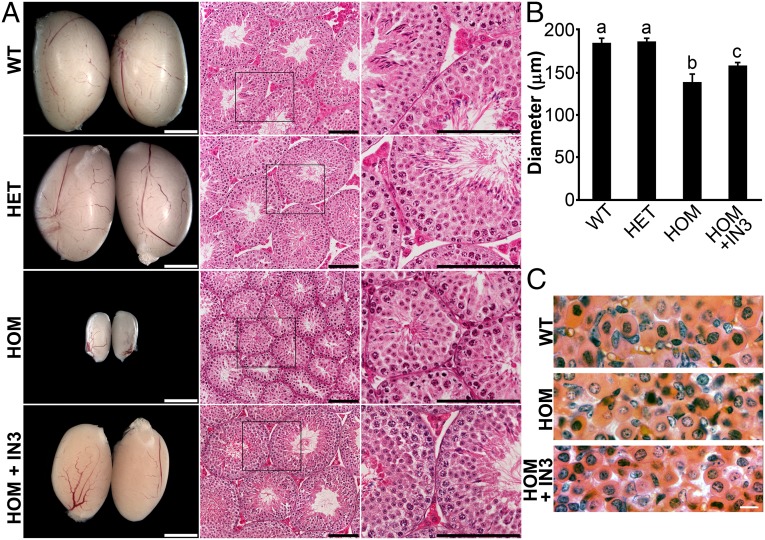
At the gross and microscopic levels, the testes of E90Kneo heterozygotes were indistinguishable from that of WT. The penetrance of the HH phenotype in E90Kneo homozygotes was variable. Whereas 100% of homozygotes exhibited reduced testis weight and infertility, ∼50% exhibited reduced numbers of elongated spermatids in H&E-stained sections (Fig. 3A and Table S1). After 30 d of IN3 infusion, testis size had increased and histology looked equivalent to WT as evident by larger seminiferous tubules harboring elongated spermatids and eosin-enriched Leydig cells (Fig. 3A).
Fig. 3.
(A) Male phenotype. Testes from the indicated genotypes were collected at 90 d of age, imaged with a stereomicroscope, and histology was examined by H&E staining. WT and E90Kneo/+ (HET) testes were indistinguishable in size, appearance, abundance of Leydig cells, and presence of spermatozoa in the seminiferous tubules. The testes of E90Kneo/E90Kneo (HOM) males were smaller and exhibited varying degrees of hypogonadism. There were few to no eosin-stained Leydig cells or elongated spermatids. Treatment of E90Kneo/E90Kneo males with pharmacoperone IN3 for 30 d (HOM + IN3) resulted in increased testis size and restored spermatogenesis. The stereomicroscopic image is shown in Left, and the H&E-stained sections are shown on Center and Right. (B) Spermatogenic activity was assayed by measuring seminiferous tubule diameter (SI Materials and Methods and Table S1). Mean seminiferous tubule diameter was not different between WT and E90Kneo heterozygotes. Seminiferous tubule diameter was reduced in E90Kneo/E90Kneo males. IN3 treatment increased the mean seminiferous tubule diameter of E90Kneo/E90Kneo males, but did not restore it to WT levels. Significant differences (P < 0.05) are denoted by the lowercase letters above each bar. Equivalent means have the same letter; different letters indicate statistically significant differences (a, b, c). Error bars show SEM. For WT, E90Kneo/+, E90Kneo/E90Kneo, and IN3 groups, n = 6, 12, 10, and 12, respectively. (C) Pituitary morphology. Images of the anterior pituitary from WT, E90Kneo/E90Kneo, and IN3-rescued E90Kneo/E90Kneo males look similar. (Scale bars: A Left, 2 mm; A Center and Right, 0.1 mm; C, 10 μm.)
We quantified alterations in testis histology by measuring the diameter of the seminiferous tubules in H&E-stained sections. The mean seminiferous tubule diameter of E90Kneo heterozygotes was similar to that of WT animals. Mean seminiferous tubule diameter was reduced for E90Kneo homozygotes. IN3 treatment increased the mean seminiferous tubule diameter for E90Kneo homozygotes but did not restore it to the full WT diameter size (Fig. 3B).
Pharmacoperone Infusion Rescues Sperm Morphology.
Because the mouse jacket interfered with mating studies, we isolated sperm from the epididymis of IN3-rescued E90Kneo/E90Kneo males and performed sperm analysis and in vitro fertilization (IVF). Sperm from cauda epididymides were isolated for IVF and analysis of total count, viability, motility, forward progression, and general morphology. Sperm from E90Kneo homozygotes possessed defects, including reduced concentration, viability, and motility, and abnormal morphologies, including headlessness and looped tails. IN3 rescue resulted in increased sperm concentration, a higher percentage of normal sperm, and less looped tails (Table 1). Sperm from three IN3-treated animals were used for IVF and produced blastocysts that were implanted into a surrogate female and resulted in a mouse pup with the expected (heterozygous) genotype.
Table 1.
Evaluation of sperm morphology and motility
| Parameter | E90Kneo/E90Kneo | E90Kneo/E90Kneo + IN3 | WT (IVF laboratory average) |
| Sperm concentration, x 106/mL | 1.1 ± 0.2 | 2.5 ± 0.2* | 3.0 |
| Viability, % | 65 ± 0.0 | 66.7 ± 1.7† | 75 |
| Total motile, % | 50 ± 0.0 | 51.7 ± 1.7† | 60 |
| Grade A motility, % | 8 ± 1.2 | 30 ± 0.0† | 30 |
| Normal sperm, % | 22.5 ± 4.8 | 56.3 ± 9.1* | 75 |
| Headless, % abnormal sperm | 8.0 ± 2.1 | 13.7 ± 3.3 | 0 |
| Thin head, % abnormal sperm | 10.8 ± 4.7 | 9.0 ± 1.5 | 15 |
| Looped tail, % abnormal sperm | 59.0 ± 3.9 | 21.0 ± 6.0* | 9 |
Comparisons were made between K90neo/K90neo and IN3 groups by t test. Data are presented as mean ± SEM. For K90neo/K90neo and IN3 groups, n = 4 and 3, respectively. Significant improvement,
P < 0.05.
The t test could not be performed because variance = 0 for at least one group.
Pituitaries of WT, Mutant, or Rescued Mutant Lack Hyperfunctioning Gonadotropes.
The pituitaries of E90Kneo/E90Kneo males do not show castration cells (11), which form after gonadectomy in response to elevated stimulation by GnRH (Fig. 3C). The likely explanation for the absence of these cells is that E90Kneo/E90Kneo males lack PM GnRHRs and cannot respond to stimulation by the elevated endogenous GnRH associated with loss of steroidal feedback. This result is similar to that observed following blockade of endogenous GnRH with anti-GnRH antibodies in castrate rodents (11).
Pharmacoperone Infusion Increases StAR and CYP11A1 Protein Levels in the Testis.
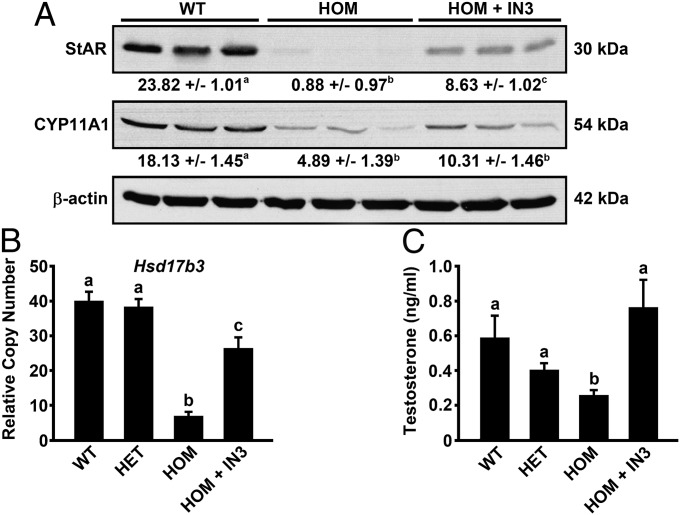
Steroidogenic acute regulatory protein (StAR) mediates cholesterol transfer within the mitochondria, the rate-limiting step in the production of steroid hormones; its production in the testes is promoted by circulating levels of LH. We examined StAR protein levels in three testes each of WT, E90Kneo homozygotes, and IN3-treated E90Kneo homozygotes by Western blotting. Compared with WT, StAR protein was markedly decreased in E90Kneo homozygotes. The loss was substantially reversed by IN3 treatment (Fig. 4A).
Fig. 4.
(A) Comparison of StAR and CYP11A1 protein expression in the testes of WT, E90Kneo/E90Kneo (HOM), and IN3-rescued E90Kneo/E90Kneo males (HOM +IN3). Fifty micrograms of protein from each animal was analyzed by Western blot. Protein levels of β-actin were used as a loading control. Western results were quantitated by densitometry. The mean values obtained from densitometry ± SEM are reported below each blot. These data were subject to ANCOVA by using β-actin values as the covariable. Statistically different means (P < 0.05) are denoted by the superscript letter following each value. Equivalent means have the same letter; different letters indicate statistically significant differences (a,b,c). When animals received IN3, they received 6-h pulses every 3 d for 30 d. (B) Hsd17b3 qPCR. Real-time PCR was performed by using total RNA extracted from the testis. Data were analyzed by the ΔΔCT method and reported as copy number relative to that of Gapdh. WT and E90Kneo heterozygotes, which are both phenotypically normal, exhibited similar levels of Hsd17b3 mRNA. E90Kneo/E90Kneo males, which exhibit hypogonadism, had reduced Hsd17b3 mRNA. Hsd17b3 expression was increased in E90Kneo/E90Kneo males following pharmacoperone treatment. For WT, E90Kneo/+, E90Kneo/E90Kneo, and IN3-rescued E90Kneo/E90Kneo, n = 9, 14, 17, and 8, respectively. (C) IN3 restored testosterone levels in E90Kneo/E90Kneo males. The serum concentration of testosterone was measured by RIA. E90Kneo/E90Kneo males exhibited less serum testosterone than heterozygous and WT males. Testosterone levels were restored in E90Kneo/E90Kneo males after pharmacoperone treatment. Significant differences (P < 0.05) are denoted by the lowercase letters above each bar. Equivalent means have the same letter; different letters indicate statistically significant differences (a,b,c). Error bars show SEM for the mean of n ≥ 3.
Side-chain cleavage cytochrome (P450scc or CYP11A1) is a mitochondrial enzyme that catalyzes conversion of cholesterol to pregnenolone, the first reaction in the process of steroidogenesis in all mammalian tissues that specialize in the production of various steroid hormones. Similar to StAR, expression of this enzyme was reduced in E90Kneo homozygotes as assessed by Western blotting (Fig. 4A). CYP11A1 protein expression appeared to increase following IN3 treatment, but our densitometric analysis showed no significant effect of IN3 (Fig. 4A). The more modest reduction in CYP11A1 protein levels may reflect the observation that this enzyme is always active, but its activity is limited by the supply of cholesterol in the inner mitochondrial membrane. The transfer of cholesterol from the outer to the inner mitochondrial membrane is the rate-limiting step in steroid production, a process mediated by StAR. Upon stimulation of a steroidogenic cell, the expression of StAR is rapidly increased and is thus available to transfer cholesterol to the inner membrane for conversion to pregnenolone.
Pharmacoperone Infusion Improves Steroid Dehydrogenase 17β Gene Expression and Serum Testosterone.
Testes were evaluated by quantitative real-time PCR for mRNA levels of hydroxyl steroid dehydrogenase 17β3 (Hsd17b3) (Fig. 4B), an enzyme that catalyzes the conversion of the poorly bioactive 17-keto steroids to the highly bioactive 17β-hydroxy steroids including testosterone (12). Levels were similar between WT and heterozygotes. Levels in homozygotes were markedly reduced. IN3 treatment increased Hsd17b3 expression in homozygotes, but not to WT levels. Despite the “subnormal” level of this rate-limiting enzyme, circulating levels of testosterone were improved in IN3-treated homozygous mutants (Fig. 4C).
Discussion
Comparison of Gnrhr Mouse Mutants Reveals the Consequences of Fine Alteration in GnRHR PM Expression.
We created the GnrhrE90K allele in mice to mimic the human disease HH and to generate a small animal model to study the efficacy of pharmacoperone treatment in vivo. In humans, mutant E90K can produce HH when homozygous. It is completely retained by the QCS and acts as a dominant negative to WT GnRHR (13, 14). In cell culture, mouse E90K is also recognized as misfolded by the QCS and retained in the ER. For both human and mouse E90K, PM localization and signal transduction can be restored with pharmacoperone IN3 (7). With this knowledge, we generated mice harboring GnrhrE90K to test the ability of pharmacoperones to correct a disease resulting from GPCR misfolding in vivo. Knowing that human E90K is a dominant negative and can produce HH when recessive, we constructed a gene targeting vector with neo in reverse orientation to the transcriptional direction of Gnrhr. If mouse E90K acted as a dominant negative, the idea was that this allele would suppress expression of the E90K mutant, prevent infertility, and allow us to maintain the line. There is support for this strategy in the literature (15, 16). Neo could be removed from the locus with cre recombinase to induce HH before experimentation. However, mice heterozygous for E90K alone or E90Kneo are phenotypically normal and fertile (17).
Interestingly, E90K homozygotes exhibit mild HH. Males have reduced testis size but are fertile. Females are infertile and fail to ovulate (17). These data suggested that mutant E90K is only partially retained by the QCS in mice and that PM-expressed E90K can respond to ligand (GnRH) with gonadotropin release. Albeit, the amount of gonadotropin released is not sufficient to stimulate ovulation.
These phenotypes are much milder than our previously characterized L117P allele. GnrhrL117P homozygous males have very small testes with a complete absence of spermatogenesis. L117P homozygous females have small, immature ovaries with no follicular growth past the primary follicle stage (9), whereas heterozygotes are fertile and phenotypically normal. Unfortunately, mutant L117P is not rescuable by pharmacoperone, presumably due to the covalent conformational change caused by the leucine to proline substitution.
Mice homozygous for E90Kneo, in which neo was not removed from the first intron, exhibit HH that is much more severe than E90K alone, but less than that of L117P. Both males and females are infertile. Testis size is smaller than E90K alone but larger than L117P. Histological sections show varied penetrance as to the degree of spermatogenesis, but testis histology is never as severely compromised as that observed for L117P. These phenotypic observations, combined with our Gnrhr gene expression data, support the conclusion that a portion of mutant E90K is retained by the QCS and the level of Gnrhr transcription is reduced by neo. This scenario presumably creates a mouse with a low level of PM-localized E90K and a “store” of QCS-retained E90K in the ER that can be mobilized to the cell surface by IN3. Importantly, E90Kneo mice exhibit infertility and a degree of HH similar to that seen in human patients and, thus, are a good model for pharmacoperone therapy.
GnRHR E90K Is Functional When Routed to the Cell Surface.
Previous work in cell cultures demonstrated that both human and mouse GnRHR E90K could be routed to the cell surface via pharmacoperone treatment. Once correctly routed to the PM, the mutant receptor responded to ligand by initiating signal transduction (9). This study extends these findings in two ways. First, collective evidence from comparing the phenotypes of our three Gnrhr mutants suggests that only a portion of E90K is retained in vivo, so any gonadotropin release is the effect of E90K localized to the gonadotrope PM. Second, in hypogonadotropic hypogonadal E90Kneo homozygotes, IN3 infusion increases LH release and improves testis function. These data support the conclusion that E90K recognizes its ligand (GnRH) and functions normally when correctly routed to the PM in vivo. This observation bodes well for the use of pharmacoperone therapy to correct diseases caused by GPCR misfolding.
GnrhrE90K Mice Are a Model for Pharmacoperone Therapy.
In this study, we show the utility of pharmacoperone drugs to correct disease by rescuing PM expression rescue of a misfolded GPCR. Previous work using mouse models of lysosomal storage diseases demonstrated that pharmacoperone treatment can stabilize inactive misfolded soluble lysosomal enzymes and improve their function (18–20). These studies provided proof of principle that pharmacoperones can stabilize a misfolded protein in vivo. Furthermore, a nonpeptide V1a antagonist was able to attenuate symptoms of diabetes insipidus in patients with missense mutations to the AVPR2 gene that cause misfolding (21). Similar to our previous work with GnRHR, V2R-specific pharmacoperones were able to restore cell surface expression and signaling of misfolded V2R mutants in COS-1 cells (21). In this study, we expanded on this line of research by generating and characterizing a genetic mouse model (GnrhrE90K) to directly test the usefulness of pharmacoperone therapy to restore PM localization and function of a GPCR in vivo. Benefiting from our mouse model, we were able to show mechanistically that delivery of a pharmacoperone to the target organ (anterior pituitary) increased routing of the misfolded GPCR (GnRHR E90K) to the gonadotrope cell surface, increased receptor activity (assayed by LH release), improved testis function (spermatogenesis and testosterone production), and restored fertility. These data support the utility of pharmacoperones for the treatment of diseases caused by protein misfolding.
Pharmacoperone therapy is a unique approach for the treatment of disease when the underlying pathology is due to protein misrouting. The pharmacoperone used in this study (IN3) shows high specificity for the GnRHR (5, 22–25) and acts by creating a surrogate bridge (D98-pharmacoperone-K121) between transmembrane segments 1 and 2 of this GPCR, enabling the mutant receptor to pass the QCS and traffic to the PM, restoring its activity (22, 25).
Four different chemical classes of pharmacoperones also rescue most of the HH-associated GnRHR mutants, even when the sites of mutations are distant to one another. These data suggest that these drugs stabilize the core component of the protein required for correct trafficking (4). Further, the ability of individual pharmacoperones to rescue many different mutants of the GnRHR (4) is therapeutically advantageous because this observation suggests that each mutant of a particular protein will not require a separate drug. The observation that pharmacoperones rescue newly synthesized misfolded mutants as well as those that have previously accumulated in the ER (26) suggests that it is not necessary to have this drug present at the time of protein synthesis, an observation that will facilitate the timing of therapeutic administration.
Pharmacoperone rescue of misfolded molecules potentially has applicability to a broad range of proteins that cause diseases responsible for cystic fibrosis, nephrogenic diabetes insipidus, disorders of vision, digestion, and neurodegeneration, and hypogonadotropic hypogonadism (1). A short-term study in patients suffering from nephrogenic diabetes insipidus (caused by ER retention of a mutant of the V2 receptor) was conducted with pharmacoperone drugs (21). Although the drug showed efficacy, the use of oral administration (and the limited number of dose regimes in a small number of patients) did not enable optimization of the pattern of pulsatile administration. Pharmacoperones for the V2, GnRHR, and other targets have been identified from antagonist screens. The present work shows that considerable efficacy can be obtained for protein rescue if the pattern of administration is carefully selected. In the present study, the treatment corrected deficits of serum LH and androgen levels and enabled the elaboration of morphologically correct and functional sperm. New high-throughput screens (27, 28) for pharmacoperones will likely lead to additional interest in this approach.
Materials and Methods
Development, Breeding, and Genotyping of the Mouse Mutant.
These methods are described in SI Materials and Methods. Animal procedures were approved by the Institutional Animal Care and Use Committees of either the University of Texas M.D. Anderson Cancer Center or Oregon Health Science University, depending where the specific work was done.
Endocrine Responses, Tissue Collection, Sperm Assessment, IVF, Endocrine Histology, Immunoblotting and Morphology, and Statistics.
These methods are described in SI Materials and Methods.
Surgery and Infusion.
Insertion of the catheter and infusion is described in described in SI Materials and Methods. These procedures were approved by the Oregon Health Science University Institutional Animal Care and Use Committee.
Supplementary Material
Acknowledgments
We thank Drs. Barry Zirkin, Marvin L. Meistrich, Richard Stouffer, Patricia Hinkle, Randy L. Johnson, Ilpo Huhtaniemi, and Hector Chemes for comments. This work was supported by National Institutes of Health Grants OD012220, DK85040, and OD011092 (to P.M.C.), NS061800 (Sue Aicher, Oregon Health and Science University Neuroscience Imaging Center), HD030284 (to R.R.B.), CA16672 (veterinary resources), HD063276, HD057121, and HD059946 (to S.M.), and HD17481 (to D.M.S.); the Ben F. Love Endowment (to R.R.B.); American Heart Association Grant 12BGIA11860006 (to M.D.S.); the Texas Heart Institute (M.D.S.); and Robert A. Welch Foundation Grant B1-0028 (to D.M.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. W.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315194110/-/DCSupplemental.
References
- 1.Castro-Fernández C, Maya-Núñez G, Conn PM. Beyond the signal sequence: Protein routing in health and disease. Endocr Rev. 2005;26(4):479–503. doi: 10.1210/er.2004-0010. [DOI] [PubMed] [Google Scholar]
- 2.Morello JP, Petäjä-Repo UE, Bichet DG, Bouvier M. Pharmacological chaperones: A new twist on receptor folding. Trends Pharmacol Sci. 2000;21(12):466–469. doi: 10.1016/s0165-6147(00)01575-3. [DOI] [PubMed] [Google Scholar]
- 3.Conn PM, Ulloa-Aguirre A, Ito J, Janovick JA. G protein-coupled receptor trafficking in health and disease: Lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev. 2007;59(3):225–250. doi: 10.1124/pr.59.3.2. [DOI] [PubMed] [Google Scholar]
- 4.Janovick JA, et al. Structure-activity relations of successful pharmacologic chaperones for rescue of naturally occurring and manufactured mutants of the gonadotropin-releasing hormone receptor. J Pharmacol Exp Ther. 2003;305(2):608–614. doi: 10.1124/jpet.102.048454. [DOI] [PubMed] [Google Scholar]
- 5.Janovick JA, Maya-Nunez G, Conn PM. Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: Misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab. 2002;87(7):3255–3262. doi: 10.1210/jcem.87.7.8582. [DOI] [PubMed] [Google Scholar]
- 6.Seminara SB, Hayes FJ, Crowley WF., Jr Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann’s syndrome): Pathophysiological and genetic considerations. Endocr Rev. 1998;19(5):521–539. doi: 10.1210/edrv.19.5.0344. [DOI] [PubMed] [Google Scholar]
- 7.Janovick JA, et al. Regulation of G protein-coupled receptor trafficking by inefficient plasma membrane expression: Molecular basis of an evolved strategy. J Biol Chem. 2006;281(13):8417–8425. doi: 10.1074/jbc.M510601200. [DOI] [PubMed] [Google Scholar]
- 8.Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125(6):769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- 9.Pask AJ, et al. A novel mouse model of hypogonadotrophic hypogonadism: N-ethyl-N-nitrosourea-induced gonadotropin-releasing hormone receptor gene mutation. Mol Endocrinol. 2005;19(4):972–981. doi: 10.1210/me.2004-0192. [DOI] [PubMed] [Google Scholar]
- 10.Janovick JA, Conn PM. Salt bridge integrates GPCR activation with protein trafficking. Proc Natl Acad Sci USA. 2010;107(9):4454–4458. doi: 10.1073/pnas.0914261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arimura A, Shino M, de la Cruz KG, Rennels EG, Schally AV. Effect of active and passive immunization with luteinizing hormone-releasing hormone on serum luteinizing hormone and follicle-stimulating hormone levels and the ultrastructure of the pituitary gonadotrophs in castrated male rats. Endocrinology. 1976;99(1):291–303. doi: 10.1210/endo-99-1-291. [DOI] [PubMed] [Google Scholar]
- 12.Saloniemi T, Jokela H, Strauss L, Pakarinen P, Poutanen M. The diversity of sex steroid action: Novel functions of hydroxysteroid (17β) dehydrogenases as revealed by genetically modified mouse models. J Endocrinol. 2012;212(1):27–40. doi: 10.1530/JOE-11-0315. [DOI] [PubMed] [Google Scholar]
- 13.Brothers SP, Cornea A, Janovick JA, Conn PM. Human loss-of-function gonadotropin-releasing hormone receptor mutants retain wild-type receptors in the endoplasmic reticulum: Molecular basis of the dominant-negative effect. Mol Endocrinol. 2004;18(7):1787–1797. doi: 10.1210/me.2004-0091. [DOI] [PubMed] [Google Scholar]
- 14.Leaños-Miranda A, Ulloa-Aguirre A, Ji TH, Janovick JA, Conn PM. Dominant-negative action of disease-causing gonadotropin-releasing hormone receptor (GnRHR) mutants: A trait that potentially coevolved with decreased plasma membrane expression of GnRHR in humans. J Clin Endocrinol Metab. 2003;88(7):3360–3367. doi: 10.1210/jc.2003-030084. [DOI] [PubMed] [Google Scholar]
- 15.Arango NA, Lovell-Badge R, Behringer RR. Targeted mutagenesis of the endogenous mouse Mis gene promoter: In vivo definition of genetic pathways of vertebrate sexual development. Cell. 1999;99(4):409–419. doi: 10.1016/s0092-8674(00)81527-5. [DOI] [PubMed] [Google Scholar]
- 16.Ventura A, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445(7128):661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 17.Stewart MD, et al. Mice harboring Gnrhr E90K, a mutation that causes protein misfolding and hypogonadotropic hypogonadism in humans, exhibit testis size reduction and ovulation failure. Mol Endocrinol. 2012;26(11):1847–1856. doi: 10.1210/me.2012-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishii S, Yoshioka H, Mannen K, Kulkarni AB, Fan JQ. Transgenic mouse expressing human mutant alpha-galactosidase A in an endogenous enzyme deficient background: A biochemical animal model for studying active-site specific chaperone therapy for Fabry disease. Biochim Biophys Acta. 2004;1690(3):250–257. doi: 10.1016/j.bbadis.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda J, et al. Chemical chaperone therapy for brain pathology in G(M1)-gangliosidosis. Proc Natl Acad Sci USA. 2003;100(26):15912–15917. doi: 10.1073/pnas.2536657100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiozuka C, et al. Increased globotriaosylceramide levels in a transgenic mouse expressing human alpha1,4-galactosyltransferase and a mouse model for treating Fabry disease. J Biochem. 2011;149(2):161–170. doi: 10.1093/jb/mvq125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernier V, et al. Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. J Am Soc Nephrol. 2006;17(1):232–243. doi: 10.1681/ASN.2005080854. [DOI] [PubMed] [Google Scholar]
- 22.Conn PM, Janovick JA. Drug development and the cellular quality control system. Trends Pharmacol Sci. 2009;30(5):228–233. doi: 10.1016/j.tips.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Conn PM, Janovick JA. Trafficking and quality control of the gonadotropin releasing hormone receptor in health and disease. Mol Cell Endocrinol. 2009;299(2):137–145. doi: 10.1016/j.mce.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janovick JA, Park BS, Conn PM. Therapeutic rescue of misfolded mutants: Validation of primary high throughput screens for identification of pharmacoperone drugs. PLoS ONE. 2011;6(7):e22784. doi: 10.1371/journal.pone.0022784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janovick JA, et al. Molecular mechanism of action of pharmacoperone rescue of misrouted GPCR mutants: The GnRH receptor. Mol Endocrinol. 2009;23(2):157–168. doi: 10.1210/me.2008-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janovick JA, et al. Refolding of misfolded mutant GPCR: Post-translational pharmacoperone action in vitro. Mol Cell Endocrinol. 2007;272(1-2):77–85. doi: 10.1016/j.mce.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smithson DC, Janovick JA, Conn PM. Therapeutic rescue of misfolded/mistrafficked mutants: Automation-friendly high-throughput assays for identification of pharmacoperone drugs of GPCRs. Methods Enzymol. 2013;521:3–16. doi: 10.1016/B978-0-12-391862-8.00001-6. [DOI] [PubMed] [Google Scholar]
- 28.Conn PM, Smith E, Hodder P, Janovick JA, Smithson DC. High-throughput screen for pharmacoperones of the vasopressin type 2 receptor. J Biomol Screen. 2013;18(8):930–937. doi: 10.1177/1087057113483559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.