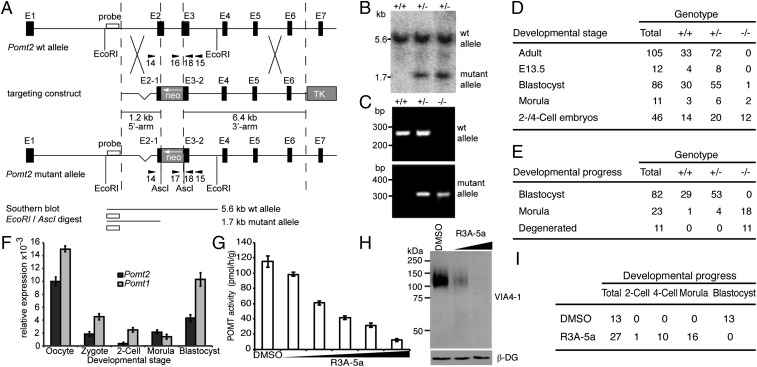
Fig. 1.
Loss of O-mannosyl glycan biosynthesis in mouse embryos. (A) Schematic representation of the Pomt2 genomic locus, targeting construct, and mutant Pomt2 allele after homologous recombination. Arrowheads indicate PCR primers. NEO, neomycin resistance gene; TK, Herpes simplex virus thymidine kinase gene. (B) Southern blot analysis of EcoRI/AscI-digested genomic DNA from targeted ES cells. Pomt2 WT (5.6 kb) and mutant (1.7 kb) alleles were detected. (C) PCR-based genotyping of preimplantation embryos. PCR products of Pomt2 WT (+/+), heterozygous (+/−), and homozygous (−/−) embryos are shown. (D and E) Genotypes and developmental stages/states of embryos (D) and in vitro-cultured embryos (E) derived from heterozygous Pomt2+/− intercrosses. (F) Relative levels of the Pomt1 and Pomt2 mRNAs during early development, as determined by quantitative RT-PCR and normalized to Ppia. Maternal Pomt1 and Pomt2 transcripts were present at high levels in oocytes but were degraded in zygotes and two-cell embryos. (G) Effect of increasing levels of R3A-5a on in vitro activity of POMT. Mouse-liver membranes were used as the source of enzyme. Reactions were supplemented with DMSO or 25 µM, 50 µM, 100 µM, 200 µM, and 400 µM R3A-5a. Mean values of three independent experiments are shown. (H) Western blot of α-DG isolated from MDCK cells cultured in the presence of 12.5 µM and 50 µM R3A-5a. The VIA4-1 monoclonal antibody was used to determine the O-mannosylation state of α-DG. β-DG levels also were assessed to confirm that protein expression and loading were equal across samples. (I) Developmental progress of WT embryos cultivated in the presence of 50 µM of the inhibitor R3A-5a.