Fig. 2.
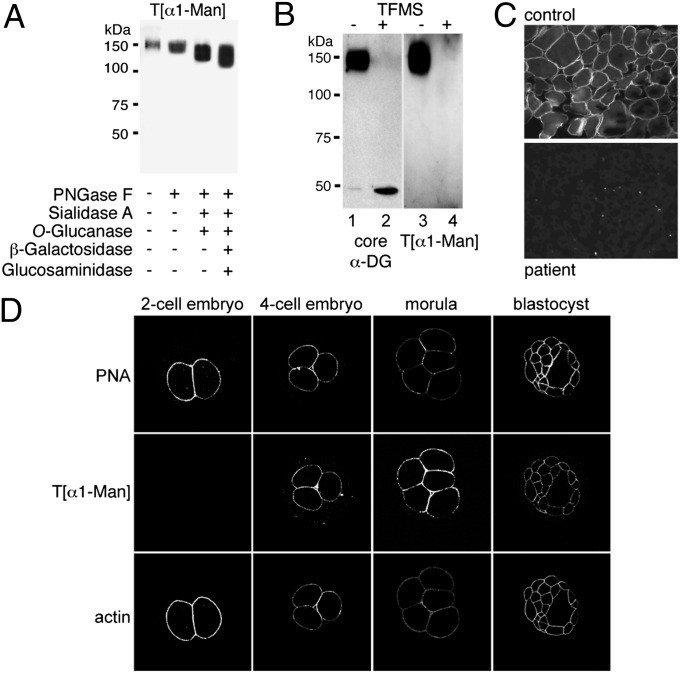
Occurrence of O-mannosyl glycans in preimplantation embryos. (A–C) Characterization of anti–T[α1-Man] antibodies. In A and B, Western blots were probed with anti–T[α1-Man] or antibodies directed against the protein core of α-DG (core αDG) as indicated. (A) α-DG–enriched glycoprotein fractions were treated with glycosidases as indicated. N-linked glycans were removed using PNGase F, and sialylated core 1 and core 3 O-linked oligosaccharides were removed by combined treatment with sialidase A and O-glucanase. Further trimming of the O-mannosyl glycan core structure Galβ1–4GlcNAcβ1–2Man-Ser/Thr was achieved by treatment with β-galactosidase and N-acetyl-β-glucosaminidase. For further details, see Fig. S3A. (B) α-DG–enriched glycoprotein fractions were treated with nonaqueous TFMS to remove attached glycans fully. Identical blots were probed. (C) Immunofluorescence staining of skeletal muscle cross-sections from an unaffected control (Upper) and a POMT1-deficient patient with Walker–Warburg syndrome (Lower) (21). Anti–T[α1-Man] immunoreactivity coinciding with the localization of O-mannosylated α-DG is observed at the sarcolemma of control muscle cells but not in the sarcolemma of the patient. (D) Detection of O-mannosyl glycans during preimplantation development. Embryos at different developmental stages were analyzed by whole-mount immunofluorescence with anti–T[α1-Man]. O-mannosyl glycans emerge at the blastomere surface at the four-cell stage. The blastomere surface was visualized using PNA, and cortical actin was visualized by staining with phalloidin.