Fig. 4.
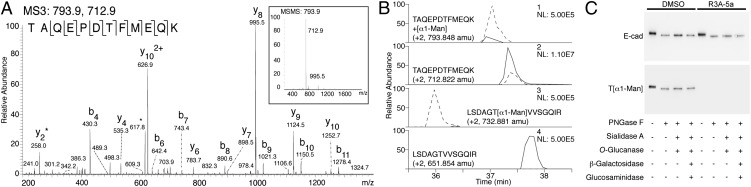
Detection of O-mannosyl glycans on E-cad. (A and B) E-cad–derived glycopeptides were analyzed by a combination of LC-MS/MS sequencing after CID and specific demannosylation using α-mannosidase as recently described (ref. 33 and SI Materials and Methods). The EC4-derived peptide TAQEPDTFMEQK was found to be modified with a single O-linked mannose residue. (A) MS3 analysis of the hexosylated peptide TAQEPDTFMEQK. (Inset) CID of a doubly charged peptide (m/z = 793.9) identified a dominant fragment ion (m/z = 712.9) formed by a neutral loss of the mass of a hexose. This fragment ion was selected for additional fragmentation leading to β and γ ions caused by backbone fragmentation of a peptide, allowing its identification. (B) Enzymatic demannosylation of the E-cad peptide TAQEPDTFMEQK (lanes 1 and 2) and a synthetic O-mannosylated control peptide LSDAGT(α1-Man)VVSGQIR (lanes 3 and 4). Peptides were treated without (dashed line) and with (solid line) α-mannosidase and were analyzed by LC-MS. Extracted-ion chromatograms of the mannosylated peptides (lanes 1 and 3) show that intensity decreased significantly upon α-mannosidase treatment. Consequently, signal intensities of the demannosylated peptides (lanes 2 and 4) increased. NL, normalized intensity level (counts per second). (C) Western blotting of affinity-purified E-cad following treatment of MDCK cells with 50 µM R3A-5a or mock treatment (DMSO), using the E-cad antibody DECMA-1 and the T[α1-Man]–specific antibody. Glycans were removed by treatment with glycosidases as indicated (for details see Fig. 2A).