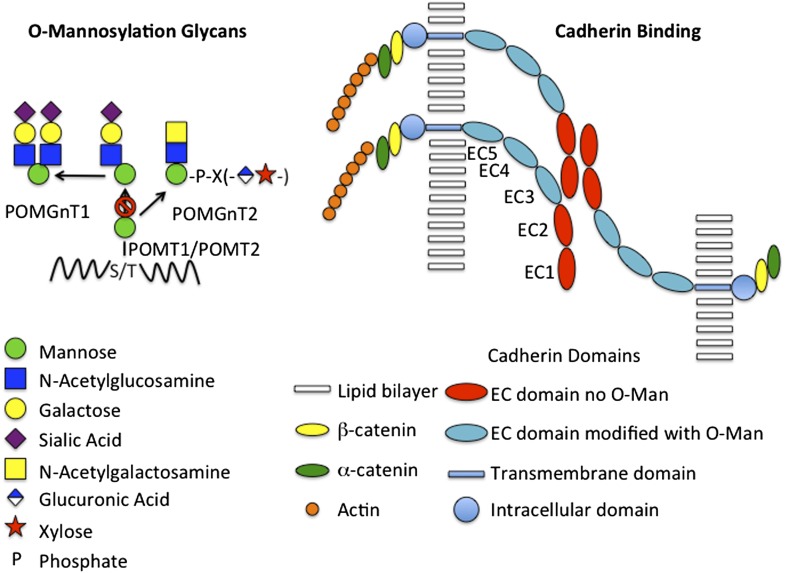
Fig. 1.
O-mannosylation glycans and cadherin binding. (Left) The structures of O-mannosylated glycans found on glycoproteins such as αDG are illustrated schematically. Man is transferred to Ser or Thr by either POMT1 or POMT2. Most often (Far Left) N-acetylglucosamine is added in β1,2-linkage by POMGnT1, yielding glycans with either one or two branches that terminate with sialic acid. Alternatively POMGnT2 can add N-acetylglucosamine in β1,4-linkage (Near Left) leading to the synthesis of a complex structure that is still under investigation. Elimination of POMGnT1 results in the presence of O-Man at all sites except those that undergo N-acetylglucosamine addition by POMGnT2 (Right). The classic cadherins form trans homodimers across apposing cells via interactions involving EC1 and EC2, which are not O-mannosylated. The EC domains more proximal to the membrane, EC3-5, are O-mannosylated and present the more distal EC domains to each other. O-mannosylation and calcium binding between the proximal EC domains may be critical for presenting the EC1 and EC2 domains in a manner that permits trans homodimer formation and adhesion. The intracellular domain interacts with actin via α- and β-catenin and is also able to modulate activation of signaling pathways. Loss of adhesion is seen in transformed cells and may increase the chances of these cells metastasizing.