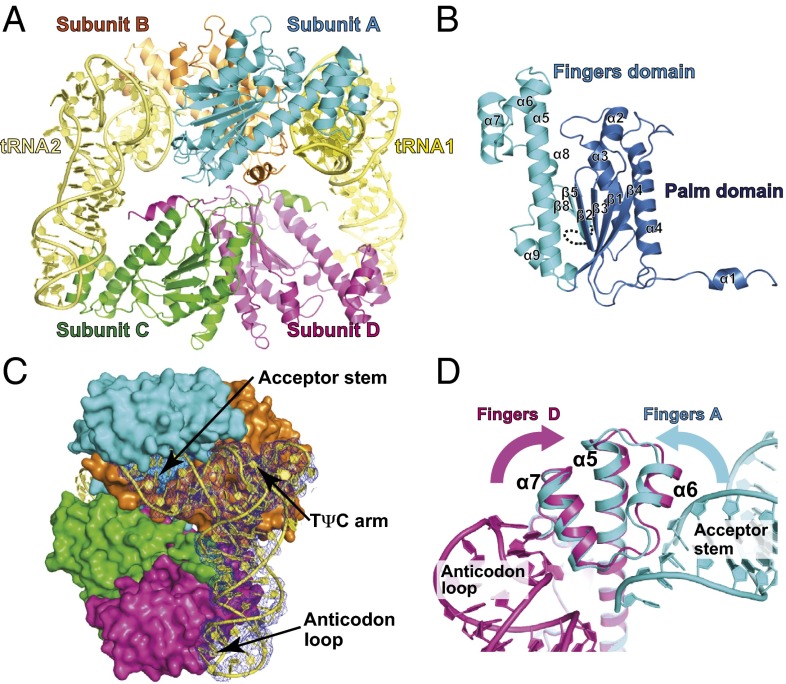
Fig. 2.
The crystal structure of the CaThg1-tRNA complex. Shown is the structural arrangement and domain organization of Thg1 in complex with tRNA. (A) The overall structure of the CaThg1-tRNA complex consists of a CaThg1 tetramer and two tRNA molecules (tRNA1 and tRNA2). The subunits of Thg1 are colored as follows: cyan, subunit A; orange, subunit B; green, subunit C; and magenta, subunit D. tRNA1 and tRNA2 are colored yellow and light yellow, respectively. (B) The domain organization of CaThg1. The palm domain (residues 1–137) and finger domain (residues 138–268) are colored in blue and cyan, respectively. (C) One tRNA molecule is recognized by cross-subunit interactions of three Thg1 molecules. The CaThg1 tetramer is displayed as a surface model. The 2mFo-DFc map for tRNA1 is colored in blue and is contoured at 1.0 σ. (D) Dual RNA-binding surface (α5, α6, and α7) of the finger domain. The helix bundle α5–α7 of subunit D with tRNA1 (magenta) was superposed onto that of subunit A (cyan). The binding of tRNA induced the conformational change of the finger domain.