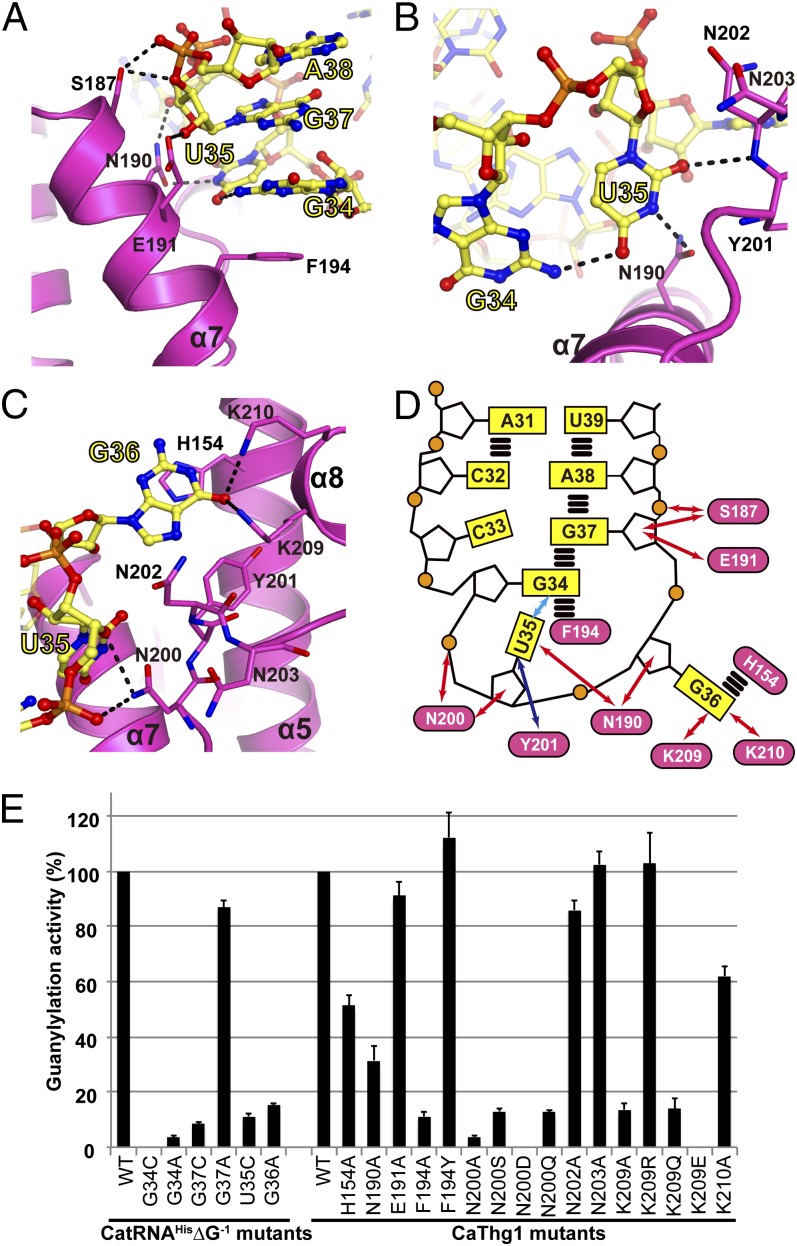
Fig. 3.
CaThg1 specifically recognizes the three anticodon bases of tRNAHis. Anticodon bases G34 (A), U35 (B), and G36 (C) are tightly coordinated by the finger domain. Amino acid residues interacting with the substrate tRNA are displayed as stick models; dashed lines indicate hydrogen bonds. (D) Schematic representation of anticodon loop recognition by the finger domain. Blue and red arrows show hydrogen bond interactions between Thg1 and the anticodon loop mediated by the protein main chains and side chains, respectively. A cyan arrow indicates RNA–RNA interactions; parallel horizontal lines represent stacking interactions. (E) Mutational analysis of the interface region between the finger domain and the anticodon loop. The rate of guanylylation activity using [α-32P]GTP, wild-type CaThg1, and wild-type CatRNAHis∆G−1 is denoted as 100. The error bars show the SD of three independent experiments.