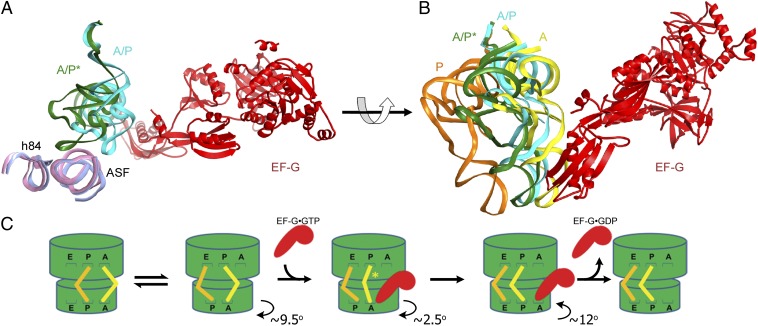
Fig. 4.
Intermediate states of tRNA translocation. (A) Structural alignment of 23S rRNA in cryo-EM structures of the EF-G-free (light pink) and EF-G-bound (light blue) pretranslocation ribosomes (both determined in this work) reveals that EF-G binding induces the movement of peptidyl-tRNA into the A/P* hybrid state described in this work. For clarity, only EF-G (red), peptidyl-tRNA, and helices 38 (the A-site finger, ASF) and 84 (h84) of 23S rRNA are shown, whereas other structural components of the ribosome are omitted. Peptidyl-tRNA bound to the A/P hybrid state in the EF-G-free ribosome is shown in cyan; peptidyl-tRNA bound to the A/P* hybrid state in the EF-G-bound ribosome complex is shown in dark green. (B) Position of the intermediate tRNA A/P* state in the pretranslocation EF-G-bound ribosome structure (dark green) relative to the A/P state of the EF-G-free hybrid-state ribosome (cyan) and classic A/A- (yellow) and P/P- (orange) tRNAs of the nonrotated, classic-state ribosome (9, 53). Superpositions in A and B were obtained by structural alignment of 23S rRNA from their respective complexes. (C) Schematic depiction of intersubunit rotation and tRNA movement during translocation. After peptidyl-transfer from the P- (orange) to the A-site (yellow) tRNA, the ribosome undergoes spontaneous fluctuations between the nonrotated (classic) and rotated (hybrid) states. Binding of EF-G•GTP (red) induces an extra 2.5° rotation of the 30S subunit (in addition to the 9.5° rotation observed in the EF-G-free hybrid-state ribosome) and shifts peptidyl-tRNA into the A/P* state. Upon subsequent clockwise rotation of the small subunit, domain IV of EF-G moves into the 30S A site and promotes the translocation of tRNAs and their associated mRNA codons on the small subunit. tRNA translocation on the small subunit is followed by dissociation of EF-G•GDP from the ribosome. The 30S head movement is not displayed for clarity. The translocation pathway may include additional intermediates that are not depicted in this scheme.