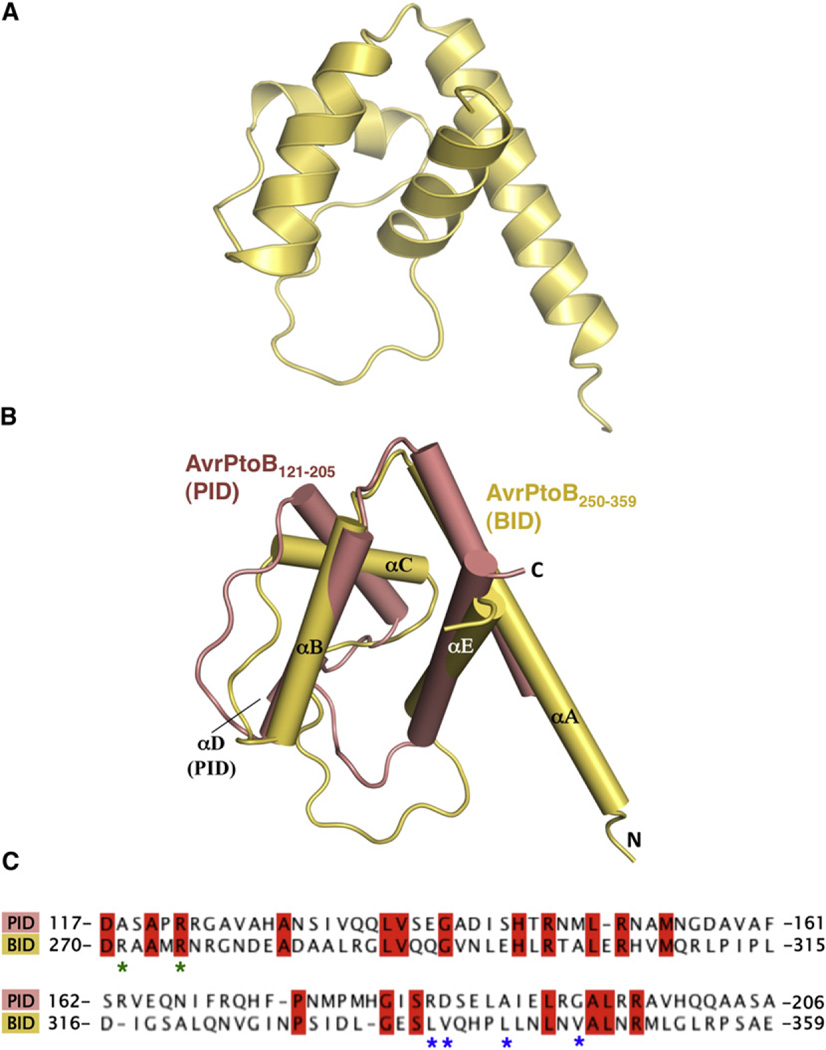
Figure 2. AvrPtoB250–359 Is a Structural Homolog of AvrPtoB121–205.
(A) BID is composed of a four-helix bundle.
(B) Structural superimposition of AvrPtoB121–205 (salmon) and AvrPtoB250–359 (light yellow). The structural elements are labeled.
(C) Primary sequence alignment of AvrPtoB121–205 and AvrPtoB250–359. Identical amino acids are highlighted in red. Asterisks indicate amino acids involved in the interaction and mutated in subsequent experiments. R271/ R275 (green asterisks) were mutated together, whereas as the others were mutated individually (see also Figure S1).