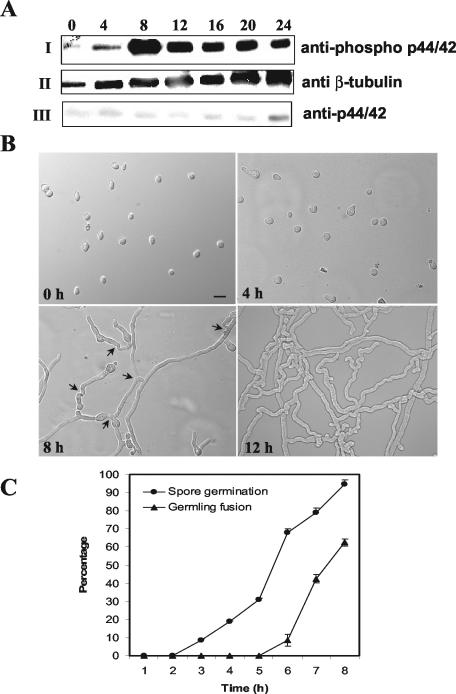
FIG. 5.
MAK-2 phosphorylation is associated with germ tube elongation and hyphal fusion between germlings. (A) Conidia from a wild-type strain (RLM 40-27) were inoculated onto a cellophane membrane layered on Vogel's minimal medium plates and incubated at 24°C. Total protein (30 μg) isolated at 0, 4, 8, 12, 16, 20, and 24 h postinoculation of conidia was used for Western blot analysis. (I) Protein extracts from the different time points probed with anti-phospho p44/42 antibodies (PhosphoPlus antibody kit; Cell Signaling Technology). (II) The blot in panel I was stripped and reprobed with anti-β-tubulin monoclonal antibodies (clone TU27; BabCo). (III) The blot in panel II was restripped and probed with anti-p44/42 antibodies. The anti-phospho p44/p42 antibodies (Cell Signaling Technology) recognize highly conserved phosphorylated T and Y residues in MAP kinases (residues 202 to 204 in Erk1 and residues 180 to 182 in MAK-2) (Fig. 1). The anti-p44/42 antibodies were raised to a peptide synthesized based on the C-terminal amino acid sequence of Erk1. The phospho-p44/42 antibody gave a stronger signal on Western blots than the anti-p44/42 antibody, presumably because of the highly conserved TEY site in MAK-2. The same experiment was repeated with unstripped blots for anti-p44/42 antibodies, and identical results were obtained. (B) DIC images of conidia and conidial germlings from the wild type (RLM 40-27) at 0, 4, 8, and 12 h postinoculation. Bar, 30 μm. (C) Quantitation of germination and hyphal fusion in conidial germlings in the wild type (RLM 40-27) over the first 8 h postinoculation. Error bars represent standard errors.