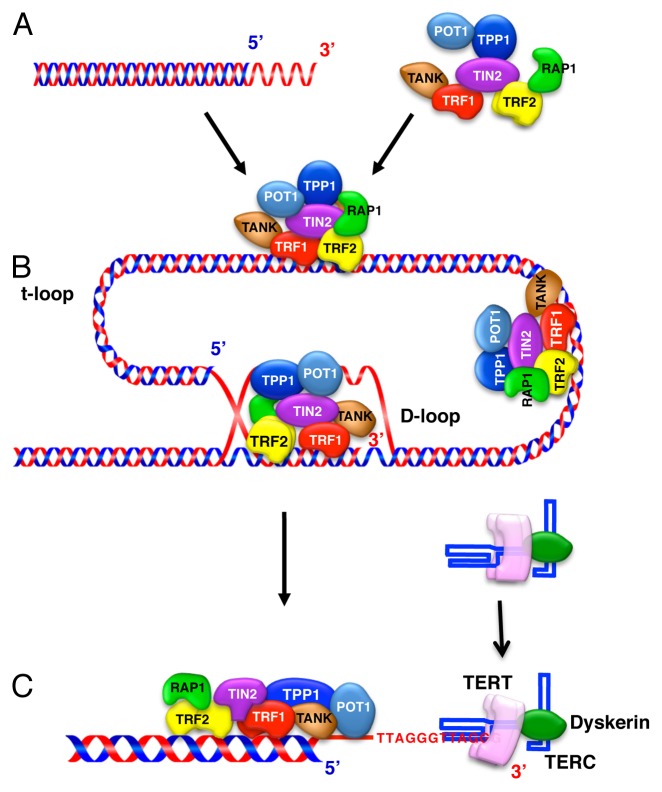
Figure 2. Schematic representation of the telomeric structure in mammalian cells. (A) Human telomeres of the chromosomal-end are composed of TTAGGG DAN repeats with the single-stranded overhang of the telomere end. (B) Telomeres are folded into a larger t-loop structure. The 3′ overhang engages in strand invasion of the adjacent duplex telomeric repeat array, forming a D-loop.114 Telomere-binding proteins, including TRF1, TRF2, RAP1, TIN2, TPP1, and POT1, form the shelterin complex, which stabilizes the t-loop structure and protects the access of telomerase to telomeric DNA.115 TRF1 can bend, loop, and pair telomeric DNA in vitro and could potentially fold the telomere. The shelterin component TRF2 mediates t-loop formation in vitro by forming a complex with RAP1, which protects telomere DNA against DNA double-strand break repair mechanisms such as non-homologous end joining, and POT1 binds both to shelterin along the duplex telomeric DNA and to the single-stranded overhang DNA. Furthermore, tankylase 1 (TANK) associates with and poly ADP ribosylates TRF1, resulting in TRF1 release from telomeric DNA and increased telomerase access to the telomere. (C) The unfolded state of the t-loop allows telomerase to access and act on the single-stranded overhang DNA. The telomerase complex extends the telomere end by the addition of TTAGGG DNA repeats using TERC as a template for the telomere repeats.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.