Abstract
Purpose
Transrectal ultrasound guided prostate biopsy results rely on physician ability to target the gland according to the biopsy schema. However, to our knowledge it is unknown how accurately the freehand, transrectal ultrasound guided biopsy cores are placed in the prostate and how the geometric distribution of biopsy cores may affect the prostate cancer detection rate.
Materials and Methods
To determine the geometric distribution of cores, we developed a biopsy simulation system with pelvic mock-ups and an optical tracking system. Mock-ups were biopsied in a freehand manner by 5 urologists and by our transrectal ultrasound robot, which can support and move the transrectal ultrasound probe. We compared 1) targeting errors, 2) the accuracy and precision of repeat biopsies, and 3) the estimated significant prostate cancer (0.5 cm3 or greater) detection rate using a probability based model.
Results
Urologists biopsied cores in clustered patterns and under sampled a significant portion of the prostate. The robot closely followed the predefined biopsy schema. The mean targeting error of the urologists and the robot was 9.0 and 1.0 mm, respectively. Robotic assistance significantly decreased repeat biopsy errors with improved accuracy and precision. The mean significant prostate cancer detection rate of the urologists and the robot was 36% and 43%, respectively (p <0.0001).
Conclusions
Systematic biopsy with freehand transrectal ultrasound guidance does not closely follow the sextant schema and may result in suboptimal sampling and cancer detection. Repeat freehand biopsy of the same target is challenging. Robotic assistance with optimized biopsy schemas can potentially improve targeting, precision and accuracy. A clinical trial is needed to confirm the additional benefits of robotic assistance.
Keywords: prostate, prostatic neoplasms, biopsy, ultrasonography, robotics
It is estimated that more than a million TRUS guided prostate biopsies are performed annually in the United States.1 The goal of current prostate biopsy is to systematically sample the prostate gland using a sextant schema or an extended variation thereof to avoid sampling limitations. However, the current freehand TRUS guided prostate biopsy technique has significant shortcomings. 1) The geometry of the exact location of the biopsy cores of the biopsy schema is poorly defined. 2) The false-negative rate of systematic TRUS guided biopsy is considered unacceptably high despite the gradually increased number of biopsy cores.2–4 3) It is impossible to precisely localize the site of a cancer core or resample the same area of interest. For example, during AS for low risk PCa, repeat sampling of the same cancerous lesion during followup is desirable to monitor cancer progression. However, repeat TRUS guided biopsies of the general areas with previously known cancer during AS frequently do not yield any cancer.5 This fact underscores our inability to consistently sample the gland with freehand TRUS guided biopsy. As a result, uncertainty regarding cancer extent and the associated anxiety often results in overtreatment. Because of these limitations, there is a critical need to improve TRUS guided biopsy.6–9
It is challenging to objectively evaluate the quality of prostate biopsy. In addition, only a few groups have addressed the distribution of biopsy cores and its relationship to the cancer detection rate.10–12 Therefore, we studied the efficacy of freehand TRUS guided biopsy and compared it with that of a robot-assisted biopsy approach. We first developed a biopsy simulation system to accurately assess the position of biopsy cores in vitro. Using that biopsy simulation system, 5 experienced urologists measured the targeting error using a freehand TRUS probe vs a robot-assisted procedure using the TRUS robot that we developed to manipulate the TRUS probe. We compared the accuracy and precision of biopsy targeting at 6 biopsy sessions by urologists and the TRUS robot. Finally, we estimated the probability of detecting significant PCa with a given number of cores.
MATERIALS AND METHODS
Biopsy Simulation System
A biopsy simulation system was composed of a custom-built pelvic mock-up and the Polaris® optical tracking system (fig. 1, A). The pelvic mock-up included a 24 cm3 model prostate fabricated from gelatin and a cavity simulating the rectum. The mock-up was molded with precisely defined geometry to standardize the model prostate position and configuration in the box. We then defined the gold standard targets as 12 points arranged as usual in an extended sextant biopsy schema in the gland. They were separated from each other by at least 10 mm.
Figure 1.
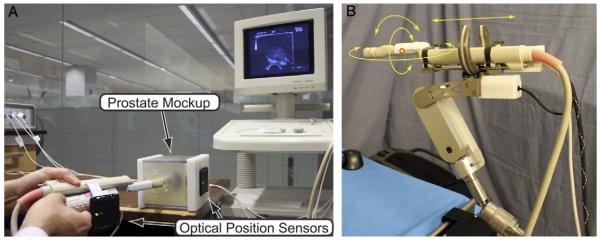
A, biopsy simulation system with prostate mock-up and optical tracking system. B, TRUS robot supporting TRUS probe.
The optical tracking system was configured to estimate the locations of the actual biopsy cores. One active (6 df) optical tracking marker was assembled on the mock-up box. Two active (6 df) markers were placed on the TRUS probe handle to measure its location and orientation. One passive (3 df) marker was placed on the biopsy needle shaft to measure needle insertion depth (fig. 1, A).
TRUS Robot
The TRUS robot is a robotic device that can hold and manipulate a TRUS probe, as described in prior studies (fig. 1, B).13,14 The TRUS robot includes software that can generate positional data and images for ultrasound scanning, 3-D reconstruction and navigation.
Experimental Protocol
Five experienced urologists performed simulated biopsy procedures with freehand TRUS probe manipulation. The same procedure was also performed with robotic assistance. All followed the usual 12-core sextant biopsy schema on 6 mock-ups (left/right × medial/lateral × apex/mid/base). A printed plan of the 12-core distribution was provided to the urologists during the simulated biopsy procedures for a uniform understanding of the desired biopsy schema. Positional data from the optical tracking system were processed to estimate the locations of biopsy cores relative to the prostate. Targeting error was calculated by measuring the 3-D distance in mm between the gold standard target and the actual biopsy target.
For repeat biopsy evaluations, our intent was to simulate the clinical scenario of consistently aiming at the same targets on repeat biopsies in the same patient. Repeat biopsy error across multiple biopsy attempts was assessed by measuring the accuracy and precision of targeting the same target in the 6 identical mock-ups. For each gold standard target, we generated a minimum enclosing sphere containing all of the centers of the actual 6 cores. Accuracy was defined as the distance between the center of each minimum enclosing sphere to its corresponding gold standard point. Precision, also known as repeatability, was defined as the diameter of this minimum enclosing sphere. In an ideal setting, a minimum enclosing sphere should have its center close to the gold standard point (high accuracy) and have a small diameter (high precision).
Significant PCa Detection Rate Modeling
For simplicity in calculating the significant tumor detection rate, a capsule modeling approach was used based on prior study of a 2-dimensional model.11,15,16 We also incorporated biopsy needle depth and direction in our 3-D capsule model. We assumed that tumors are spherical. We defined the threshold of significant tumor size as 0.5 cm3 (radius 4.924 mm).17 In the capsule modeling approach, we considered the tumor sampled and significant if its center was within a capsule with a radius (4.924 mm) equal to that of the tumor (fig. 2). This approach is equivalent to intersecting a line segment (biopsy core) with a sphere (tumor) but it simplifies the geometric calculations.
Figure 2.
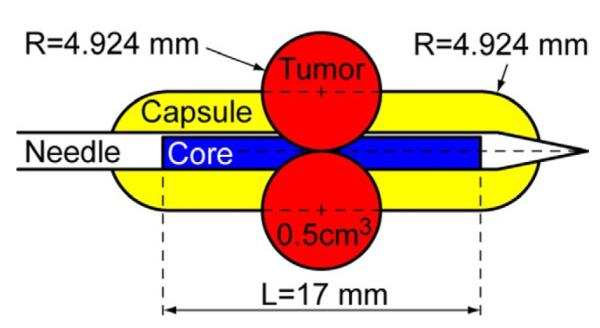
Capsule-shaped sampling volume. Capsule radius (R) is same as that of minimal significant PCa tumor (0.5 cm3) and length (L) is same as that of needle core.
For single core biopsy, sampled volume is defined as the volume of the intersection between the respective capsule and the prostate. The probability of detecting a significant tumor with this 1-core biopsy is the ratio of sampled volume to total prostate volume. For multiple core biopsy, these volumes do not simply sum up because cores may intersect each other. The probability of detecting a significant tumor with multiple cores is defined as the ratio of the combined, nonoverlapping volume of individual sampled volumes (intersection with the prostate of the union of the individual cores) to total prostate volume. Consequently, the detection rate is higher if biopsy cores sample the prostate and do not overlap.
Statistical Analysis
Biopsy error rates were compared among the urologists and the robot using ANOVA with blocks representing individual mock-ups and testers (urologists or robot). Detection rates were compared with 1-way ANOVA.18 The Dunnett test was used to adjust for multiple comparisons in pairwise tests among individual urologists and the TRUS robot.19 All analyses were performed using SAS® v. 9.2.
RESULTS
We assessed the quality of extended sextant biopsy by urologists vs TRUS robot-assisted biopsy in a) targeting error, b) repeat biopsy error and c) the estimated significant cancer detection rate.
Error
Targeting
On targeting error analysis we assessed the quality of targeted biopsy by urologists and the TRUS robot to sample a sextant biopsy target in the prostate guided by 2-dimensional ultrasound. The table shows the mean targeting errors of the 5 urologists, the urologists overall and the TRUS robot. The urologist mean targeting error was 9.0 mm.Figure 3 shows the actual centers of the cores acquired by 1 urologist (red circles) compared with the gold standard (green circles). As shown, the biopsy cores were often clustered. In contrast, the TRUS robot mean targeting error was only 1.0 mm, showing that it followed the biopsy schema more closely.
Freehand and robot-assisted repeat biopsy targeting error, accuracy and precision, and significant PCa detection rates
| Mean ± SD Targeting Error |
Mean ± SD Repeat Biopsy (mm) |
Mean ± SD % | ||
|---|---|---|---|---|
| (mm) | Accuracy | Precision | Detection | |
| Urologist No.: | ||||
| 1 | 11.4 ± 5.0 | 24.5 ± 8.7 | 11.3 ± 2.9 | 37.1 ± 5.4 |
| 2 | 10.2 ± 4.8 | 25.9 ± 6.6 | 10.5 ± 2.1 | 36.4 ± 3.9 |
| 3 | 8.5 ± 3.9 | 21.1 ± 7.4 | 11.0 ± 2.5 | 38.9 ± 3.1 |
| 4 | 7.1 ± 3.2 | 21.5 ± 7.1 | 9.1 ± 1.6 | 37.7 ± 2.7 |
| 5 | 7.9 ± 3.6 |
25.1 ± 7.3 |
8.6 ± 1.7 |
30.5 ± 3.6 |
| Av | 9.0 ± 4.4 | 23.6 ± 7.4 | 10.1 ± 2.2 | 36.1 ± 3.3 |
| Robot | 1.0 ± 0.3 | 0.6 ± 0.2 | 1.7 ± 0.3 | 43.3 ± 0.7 |
Figure 3.
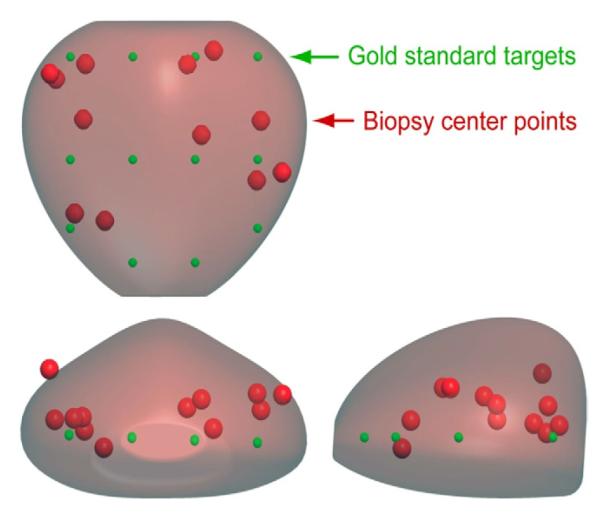
Targeting error. Two views of prostate with gold standard points (green circles) and actual biopsy cores (red circles) by urologist.
While the mean targeting error of each urologist was significantly higher than that of the robot, significant variation also existed among urologists (p <0.0001). In a prostate, mean targeting error varied significantly by biopsy location with the largest error associated with the apex than with mid/base positions (mean ± SD 10.8 ± 5.3 vs 8.1 ± 3.6 mm, p <0.0001). Finally, larger targeting errors were consistently found at medial compared to lateral positions (mean 10.4 ± 4.5 vs 7.6 ± 3.8 mm, p <0.0001).
Repeat biopsy
The mean accuracy of the urologists and the TRUS robot was 23.6 and 0.6 mm, respectively (see table). The mean precision of the urologists and the TRUS robot was 10.1 and 1.7 mm, respectively. Overall, urologist cores across multiple trials deviated widely from the gold standard, while TRUS robot cores followed the gold standard targets closely (fig. 4).
Figure 4.
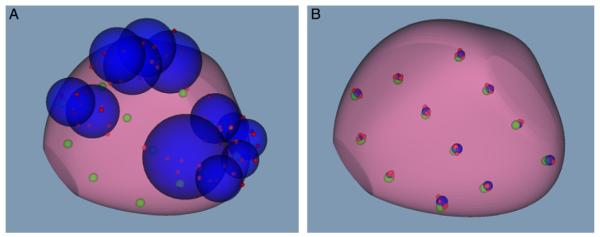
A, core distribution by 1 urologist shows gold standard (green circles) and actual cores (red circles) of all 6 mock-ups, and minimum enclosing spheres for each gold standard point (blue circles). B, same core distribution with robotic assistance.
Cancer Detection Rate
The tumor detection rates of the urologists and the robot were estimated by the capsule model (fig. 5). The average tumor detection rate of the 5 urologists was 36.1%, while the TRUS robot mean detection rate was 43.3% (see table). There was a significant difference between the urologists and the robot (p <0.0001). Significant variation was also found among the urologists (p = 0.008).
Figure 5.
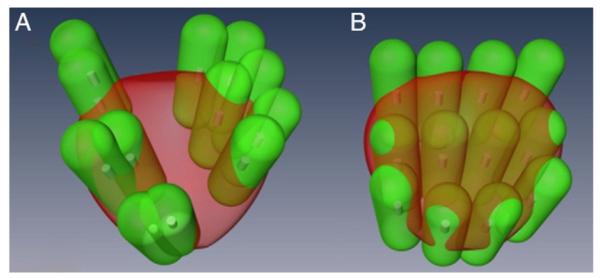
Intersection of green capsule shapes with prostate represents sampled regions. Significant tumor detection rate is estimated by ratio of total volume to prostate volume. A, urologist. B, robotic assistance.
DISCUSSION
TRUS guided freehand prostate biopsy is the current gold standard for prostate cancer diagnosis.20 The principle of the current sextant biopsy schema stipulates a uniform distribution of a set number of cores. However, to our knowledge the exact locations of the freehand biopsy cores are unknown in clinical practice.
In this study we developed an in vitro biopsy simulation model to assess the geometric distribution of systematic biopsy targeting, the accuracy and precision of repeat biopsies, and the cancer detection rate. We then compared the performance of experienced urologists with that of a robot. Our results confirm that it is challenging to use freehand TRUS guided biopsy to adequately sample the prostate or biopsy the same area repeatedly. On the other hand, improved sampling and cancer detection can be achieved with a more quantitative approach, such as robotic assistance or image guided navigation.
During biopsy targeting, even experienced urologists had difficulty with accurately sampling a specific target in the intended biopsy schema. Biopsy cores acquired by the urologists were often clustered and left a significant portion of the prostate under sampled. Experienced urologists also had significant difficulty in sampling the same target repeatedly. In a clinical context, this result implies that it is challenging for a urologist to freehand biopsy the same area twice, for example in patients enrolled in AS. Most importantly, the significant cancer detection rate was significantly lower for freehand biopsies done by urologists than for robot-assisted biopsy.
The tumor detection rate depends highly on the geometric distribution and number of biopsy cores in the schema. For example, all cores in the gold standard schema sampled prostate tissue without significant overlap (fig. 5, B). However, some capsules protruded outside the gland, thus, reducing the detection rate. In addition, the tumor detection rate with the set number of biopsy cores decreases with increasing prostate size. Therefore, biopsy schema may need to be optimized in men with different prostate sizes and shapes. Collectively, these results show that robotic or navigational assistance for biopsy must be coupled with biopsy schema that can optimize the PCa detection rate.
In the last 2 decades prostate biopsy has undergone significant changes. In 1989 Hodge et al first reported the superiority of systematic TRUS guided biopsies over digitally directed sampling.21 Since then, the number of biopsy cores has gradually increased, while targeting certain anatomical zones has been incorporated in the biopsy schema.20 In the repeat biopsy setting saturation biopsy has been developed and used with the goal of reducing false-negative results.22,23 However, there is no consensus on the optimal number of cores on saturation biopsy. In addition, there is sparse available geometric localization information on the biopsy cores.
With increasing interests in focal therapy for prostate cancer, transperineal template guided and robot-assisted biopsies have been developed.24,25 Compared to the transrectal approach, the transperineal biopsy approach can achieve improved sterility via perineal skin incision. However, transperineal biopsy approach is also associated with the need for more extensive anesthesia and with sampling limitation due to pubic arch interference.25 Most recently, magnetic resonance imaging-ultrasound fusion guided prostate biopsy, some with 3-D guidance, has been gaining interest for lesion targeted biopsy.26,27 Pursuit continues for an accurate, precise, cost-effective, safe method of prostate biopsy. The important distinction of the current study is that we report the limitations of freehand, human TRUS guided biopsy.
Several limitations deserve mention. 1) In the biopsy simulation model, we assumed that spherical tumors occur evenly in the prostate. We plan to expand our model to include nonspherical tumors in nonuniform fields with zonal differences. 2) Our prostate model was precisely standardized, unlike the highly variable size and shape of individual prostate glands in clinical practice. However, a standardized prostate model was helpful and necessary to compare biopsy efficiency by urologists and the robot. In the clinical setting accurately scanning prostates of different sizes/shapes, optimized biopsy schemas and accurate targeting are needed to further improve the PCa detection rate. 3) Since our study was performed in an in vitro model system and not in humans, we did not consider tissue deformation and needle bending, which may negatively impact biopsy.8 4) The exact coordinates of the gold standard biopsy schema were provided during robot-assisted biopsy, while the experienced urologists performed biopsy based on the B-mode ultrasound image and biopsy schema from memory and without navigational aid. As discussed, robot use or navigational assistance with optimized biopsy schema improves biopsy performance. 5) Cost analysis must be done to evaluate the overall health care benefit/cost of using a quantitative tool, such as a robot, to improve the detection of significant cancer, while decreasing overtreatment.
Despite these shortcomings, our results confirm and quantify the considerable limitations of the current freehand TRUS guided prostate biopsy that were suggested in previous reports.12,28 The robot can assist in image navigation since it can steadily hold and manipulate the imaging device (TRUS probe) accurately to generate a 3-D reconstruction image of the prostate.13 Robotic assistance can improve biopsy targeting, while potentially decreasing false-negative results by geometrically consistent sampling. This approach can 1) make primary biopsies more uniformly distributed according to the biopsy schema, 2) help sample previously unsampled regions of the prostate in men with high suspicion for cancer and yet with prior negative biopsies, and 3) help monitor cancer progression during AS by resampling known cancer foci. More accurate determination of tumor extent and progression may lead to increased participation in AS while decreasing overtreatment. Currently, a critical barrier to progress in the field of image guided targeted therapy for prostate cancer is the lack of reliable tumor localization. Precise localization of prostate cancer may also help improve focal therapy targeting.
CONCLUSIONS
Systematic biopsy with freehand TRUS guidance does not closely follow the sextant biopsy schema and may result in suboptimal sampling and cancer detection. Robot-assisted biopsy provides a potential alternative for accurate, precise sampling and may enhance the cancer detection rate if coupled with a geometrically consistent, optimal biopsy protocol.
Acknowledgments
Supported by Award R21CA141835 from the National Cancer Institute, the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins and Hitachi-Aloka Medical, Ltd.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, National Institutes of Health, Sidney Kimmel Comprehensive Cancer Center or Hitachi-Aloka Medical.
Abbreviations and Acronyms
- 3-D
3-dimensional
- AS
active surveillance
- PCa
prostate cancer
- TRUS
transrectal ultrasound
REFERENCES
- 1.Bostwick DG, Liu L, Brawer MK, et al. High-grade prostatic intraepithelial neoplasia. Rev Urol. 2004;6:171. [PMC free article] [PubMed] [Google Scholar]
- 2.Rabbani F, Stroumbakis N, Kava BR, et al. Incidence and clinical significance of false-negative sextant prostate biopsies. J Urol. 1998;159:1247. [PubMed] [Google Scholar]
- 3.Norberg M, Egevad L, Holmberg L, et al. The sextant protocol for ultrasound-guided core biopsies of the prostate underestimates the presence of cancer. Urology. 1997;50:562. doi: 10.1016/S0090-4295(97)00306-3. [DOI] [PubMed] [Google Scholar]
- 4.Svetec D, McCabe K, Peretsman S, et al. Prostate rebiopsy is a poor surrogate of treatment efficacy in localized prostate cancer. J Urol. 1998;159:1606. doi: 10.1097/00005392-199805000-00052. [DOI] [PubMed] [Google Scholar]
- 5.Tseng KS, Landis P, Epstein JI, et al. Risk stratification of men choosing surveillance for low risk prostate cancer. J Urol. 2010;183:1779. doi: 10.1016/j.juro.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Presti J., Jr Does the yield of prostate cancer biopsy and repeat biopsy justify the frequency of their use? Nat Clin Pract Urol. 2008;5:246. doi: 10.1038/ncpuro1056. [DOI] [PubMed] [Google Scholar]
- 7.Welch HG, Fisher ES, Gottlieb DJ, et al. Detection of prostate cancer via biopsy in the Medicare-SEER population during the PSA era. J Natl Cancer Inst. 2007;99:1395. doi: 10.1093/jnci/djm119. [DOI] [PubMed] [Google Scholar]
- 8.De Silva T, Fenster A, Bax J, et al. Quantification of prostate deformation due to needle insertion during TRUS-guided biopsy: comparison of handheld and mechanically stabilized systems. Med Phys. 2011;38:1718. doi: 10.1118/1.3557883. [DOI] [PubMed] [Google Scholar]
- 9.Karnik V, Fenster A, Bax J, et al. Assessment of image registration accuracy in three-dimensional transrectal ultrasound guided prostate biopsy. Med Phys. 2010;37:802. doi: 10.1118/1.3298010. [DOI] [PubMed] [Google Scholar]
- 10.Giannarini G, Autorino R, Lorenzo G. Saturation biopsy of the prostate: why saturation does not saturate. Eur Urol. 2009;56:619. doi: 10.1016/j.eururo.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 11.Kepner GR, Kepner JV. Transperineal prostate biopsy: analysis of a uniform core sampling pattern that yields data on tumor volume limits in negative biopsies. Theor Biol Med Model. 2010;7:23. doi: 10.1186/1742-4682-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mozer P, Baumann M, Chevreau G, et al. Mapping of transrectal ultrasonographic prostate biopsies: quality control and learning curve assessment by image processing. J Ultrasound Med. 2009;28:455. doi: 10.7863/jum.2009.28.4.455. [DOI] [PubMed] [Google Scholar]
- 13.Han M, Kim C, Mozer P, et al. Tandem-robot assisted laparoscopic radical prostatectomy to improve the neurovascular bundle visualization: a feasibility study. Urology. 77:502. doi: 10.1016/j.urology.2010.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoianovici D, Whitcomb L, Mazilu D. United States Patent 07021173. Remote Center of Motion Robotic System and Method. 2006 Apr 4; inventors, John Hopkins University, assignee.
- 15.Punnen S, Nam RK. Indications and timing for prostate biopsy, diagnosis of early stage prostate cancer and its definitive treatment: a clinical conundrum in the PSA era. Surg Oncol. 2009;18:192. doi: 10.1016/j.suronc.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Taira A, Merrick G, Galbreath R, et al. Performance of transperineal template-guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate Cancer Prostatic Dis. 2010;13:71. doi: 10.1038/pcan.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamey T, Freiha F, McNeal J, et al. Localized prostate cancer : Relationship of tumor volume to clinical significance for treatment of prostate cancer. Presented at American Cancer Society National Conference on Prostate Cancer; San Francisco, California. February 13–15, 1992; [DOI] [PubMed] [Google Scholar]
- 18.Neter J, Wasserman W. Applied Linear Statistical Models. RD Irwin; Homewood, Illinois: 1974. pp. 723–63. (Irwin Series in Statistics). [Google Scholar]
- 19.Dunnett CW. A multiple comparisons procedure for comparing several treatments with a control. J Amer Stat Assoc. 1955;50:1096. [Google Scholar]
- 20.Dominguez-Escrig JL, McCracken SR, Greene D. Beyond diagnosis: evolving prostate biopsy in the era of focal therapy. Prostate Cancer. 2011;2011:386207. doi: 10.1155/2011/386207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodge KK, McNeal JE, Terris MK, et al. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989;142:71. doi: 10.1016/s0022-5347(17)38664-0. [DOI] [PubMed] [Google Scholar]
- 22.Borboroglu PG, Comer SW, Riffenburgh RH, et al. Extensive repeat transrectal ultrasound guided prostate biopsy in patients with previous benign sextant biopsies. J Urol. 2000;163:158. [PubMed] [Google Scholar]
- 23.Stewart CS, Leibovich BC, Weaver AL, et al. Prostate cancer diagnosis using a saturation needle biopsy technique after previous negative sextant biopsies. J Urol. 2001;166:86. [PubMed] [Google Scholar]
- 24.Barqawi AB, Rove KO, Gholizadeh S, et al. The role of 3-dimensional mapping biopsy in decision making for treatment of apparent early stage prostate cancer. J Urol. 2011;186:80. doi: 10.1016/j.juro.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Ho H, Yuen JS, Mohan P, et al. Robotic transperineal prostate biopsy: pilot clinical study. Urology. 2011;78:1203. doi: 10.1016/j.urology.2011.07.1389. [DOI] [PubMed] [Google Scholar]
- 26.Natarajan S, Marks LS, Margolis DJ, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol Oncol. 2011;29:334. doi: 10.1016/j.urolonc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ukimura O, Desai MM, Palmer S, et al. 3-Dimensional elastic registration system of prostate biopsy location by real-time 3-dimensional transrectal ultrasound guidance with magnetic resonance/transrectal ultrasound image fusion. J Urol. 2012;187:1080. doi: 10.1016/j.juro.2011.10.124. [DOI] [PubMed] [Google Scholar]
- 28.Ukimura O, Hung AJ, Gill IS. Innovations in prostate biopsy strategies for active surveillance and focal therapy. Curr Opin Urol. 2011;21:115. doi: 10.1097/MOU.0b013e3283435118. [DOI] [PubMed] [Google Scholar]


