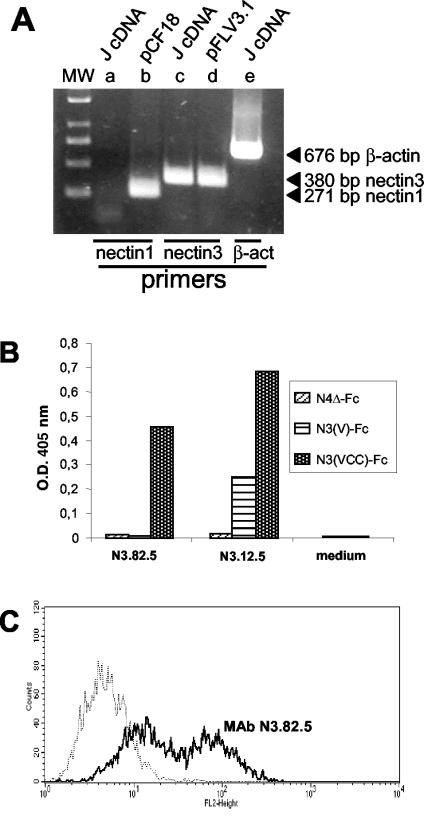
FIG. 3.
(A) RT-PCR amplification of nectin3 from retrotranscribed RNA from J cells (J cDNA). nectin3 cDNA was amplified with primers designed on human and murine nectin3 cDNA. nectin1 primers were designed on the human cDNA. Plasmids carrying human nectin1 (pCF18) or human nectin3 (pFLR3V.1) cDNA served as positive controls for nectin1 and nectin3 amplification, respectively (15, 52). The expected sizes of the amplified fragments are indicated to the right of the gel. β-act, β-actin. (B) Derivation and specificity of MAbs to nectin3. Cell culture media from the indicated hybridomas (N3.12.5 and N3.82.5 to nectin3 and medium from a negative clone [control medium]) were reacted in a sandwich ELISA with soluble nectin3 ectodomain [N3(VCC)-Fc], soluble nectin3 V domain [N3(V)-Fc], or with nectin4Δ-Fc as a negative control. Briefly, 96-well trays were coated with an antibody against the human Fc fragment (Sigma Chemical Co.) at 5 μg/ml. After the nonspecific sites were blocked with PBS containing 1% BSA, N3(VCC)-Fc, nectin3V-Fc, or nectin4Δ-Fc was added at a concentration of 10 nM, and then goat anti-mouse antibodies conjugated to peroxidase and One Step ABTS (Pierce) were added. The optical density (O.D.) at 405 nm was read. MAb N3.82.5 reacted with soluble nectin3 containing the entire ectodomain but failed to react with the soluble nectin3 containing only the V domain; therefore, it was directed against the C domains. (C) Binding of MAb N3.82.5 to unfixed J cells. J cells were reacted with MAb N3.82.5 (thick black line) (10 μg of purified IgG/ml) for 1 h at 37°C, followed by a phycoerythrin-labeled goat anti-mouse antibody (1:50) (Beckman-Coulter-Immunotech). The negative control (thin stippled line) represents reactivity to mouse IgG1 (10 μg/ml), followed by the same secondary phycoerythrin-labeled antibody. Reactivity to MAb N3.82.5 denotes cell surface expression of nectin3.