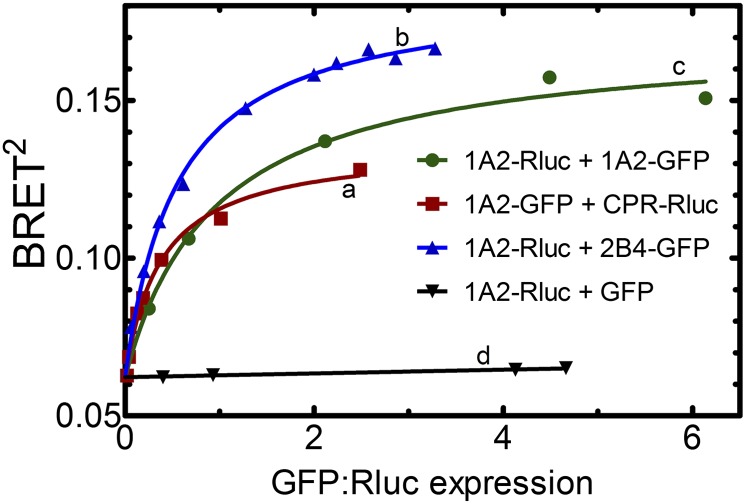
Fig. 11.
Demonstration of the existence of P450-P450 complexes in living cells using bioluminescence resonance energy transfer. Vectors were created containing CYP1A2, CYP2B4, or CPR cDNA upstream of either GFP or Rluc so that the resultant fusion proteins contained a C-terminal tag. These vectors (one -Rluc and one -GFP) were transfected into human embryonic kidney 293T cells to coexpress the fusion proteins at a variety of GFP-to-Rluc ratios. A hyperbolic increase in BRET signal (BRET2, measured as the ratio of the 510 nm GFP fluorescence/410 nm Rluc luminescence) is indicative of specific complexes between the proteins. Interaction between CYP1A2 and CPR was detected as a BRET signal after the cotransfection of CYP1A2-GFP and CPR-Rluc (curve a). Formation of homomeric CYP1A2 complexes is shown by the BRET response generated by cotransfection of CYP1A2-Rluc and CYP1A2-GFP (curve b). Formation of the heteromeric CYP1A2•CYP2B4 complex is shown by cotransfection of CYP1A2-Rluc with CYP2B4-GFP (curve c). A control curve showing a lack of complex formation was generated by cotransfecting CYP1A2-Rluc and GFP (without a P450 attached) (curve d). Curves b and d are reproduced from Reed et al., 2012.