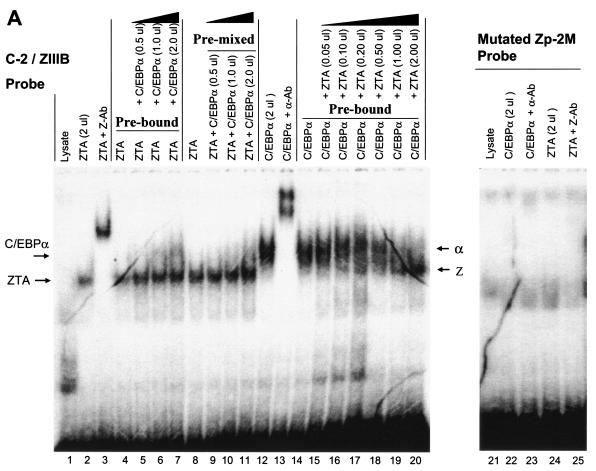
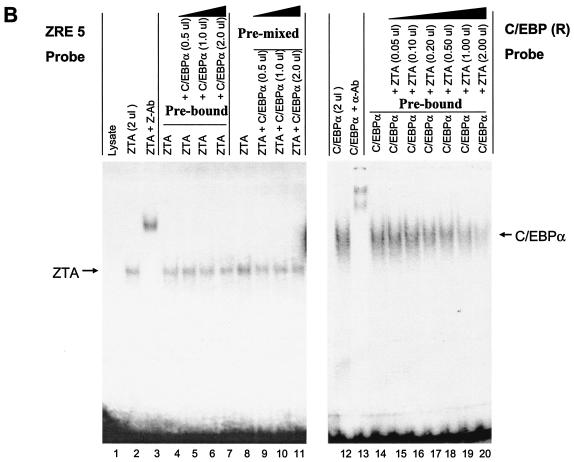
FIG. 5.
ZTA interferes with C/EBPα binding to the C/EBP C-2/ZIIIB ZRE site, but C/EBPα has no effect on ZTA binding to the same site. (A) EMSA experiment showing the following results. First, in vitro-translated ZTA (pYNC100) and C/EBPα (pYNC172a) both bind strongly and independently to the 32P-labeled C-2 probe encompassing the ZIIIB ZRE motif (lanes 1, 2, 3, 12, and 13). Second, the addition of C/EBPα at various doses (lanes 4 to 11) does not affect ZTA DNA binding to the C-2 probe, whereas in the reciprocal approach, the addition of ZTA at various doses displaces C/EBPα binding to the C-2 probe (lanes 15 to 20). Third, point mutation of the C-2/ZIIIB site inactivates binding to both proteins (lanes 21 to 25). Z-Ab, anti-ZTA antibody; α-Ab, anti-C/EBPα antibody. (B) EMSA experiment showing the results of similar dose-response mixture binding experiments with in vitro-translated C/EBPα (pYNC172a) and ZTA (pYNC100) and with consensus 32P-labeled ZRE(5) and C/EBP(R) oligonucleotide DNA probes that each bind specifically to one protein but do not cross-react with the other (lanes 1, 2, 3, 12, and 13). Again, the addition of C/EBPα failed to affect the binding of ZTA to the ZRE(5) probe (lanes 4 to 11), but the addition of ZTA gradually displaced C/EBPα binding to the C/EBP(R) probe (lanes 14 to 20).