TABLE 3.
Vinorelbine and its CYP3A4 metabolites based on liquid chromatography–MS/MS and NMR data
| ID | M+H+ | MS/MS Product Ions | Proposed Metabolite Structure |
|---|---|---|---|
| Vinorelbine | 779 | 719,701,510,469,457,323,122 | 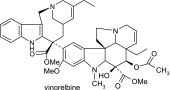 |
| M1 | 795 | 735,702,646,526,469,457,339,202,138,122 | 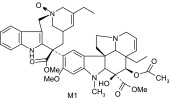 |
| M2 | 777 | 717,686,580,419,321,311 | 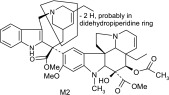 |
| M3 | 795 | 735,717,674,642,510.485,379,323,291,122 | 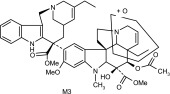 |
| M4 | 811 | 526,508,485,473,397,345,323,202 | 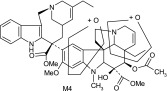 |
| M5 | 781 | 610,455,443,138,122 | 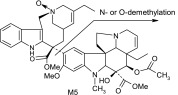 |
| M6 | 795 | 777,717,633,526,457,403,202,122,108 | 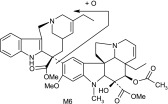 |
| M7 | 763 | 704,672,566,535,455,423,321 | 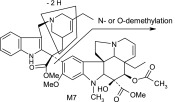 |
| M8 | 777 | 718,717,658,657,467,389 | 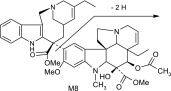 |
