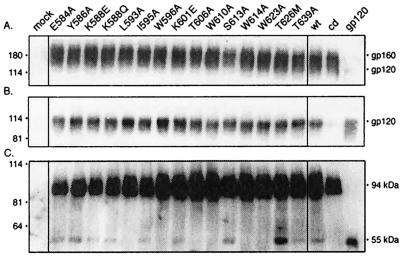
FIG. 2.
Expression of mutant envelope glycoproteins and gp120 shedding. Mutations were introduced into the HXB2 envelope glycoprotein expression plasmid by QuikChange (Stratagene, Inc.) mutagenesis (23, 34). Mutant and wild-type (wt) envelope glycoproteins were transiently expressed in COS-7 cells upon transfection with FuGene-6 reagent (Roche Molecular Biochemicals). Standards for gp160 and gp120 were obtained by expression of the cleavage-defective (cd) envelope glycoprotein and soluble gp120. (A) Cell lysates were resolved by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis, and the envelope glycoprotein was visualized by Western blot analysis with anti-gp120 monoclonal antibody Chessie B13 (1) and ECL-Plus detection (Amersham Pharmacia Biotech) (23, 34). A residuum of cell-associated gp120 is seen in cells expressing soluble gp120. (B) Cell culture supernatants were collected, and gp120 was immunoprecipitated with the anti-HIV immunoglobulin HIVIG (28). The cleavage-defective envelope glycoprotein served as a control for gp120 shedding and was subtracted for quantitation. In general, the mutant glycoproteins shed slightly more gp120 than the wild type (1.36 ± 0.28), but significant differences among the mutants were not discerned. (C) Cells were biotinylated with the membrane-impermeant agent NHS-LC-biotin (Pierce Chemical) (23, 34). Cell surface proteins were isolated with NeutrAvidin-agarose (Pierce Chemical) and deglycosylated by treatment with peptide-N-glycosidase F (New England Biolabs). The resulting polypeptides were detected by Western blot analysis with the gp120-specific monoclonal antibody Chessie 12 (1) and ECL-Plus imaging (23, 34). All mutant envelope glycoproteins were transported to the cell surface comparably to the wild type (1.20 ± 0.27). A dark image is used to display the deglycosylated gp120s. HXB2 gp160 and gp120 were deglycosylated to provide size markers (94 and 55 kDa, respectively).