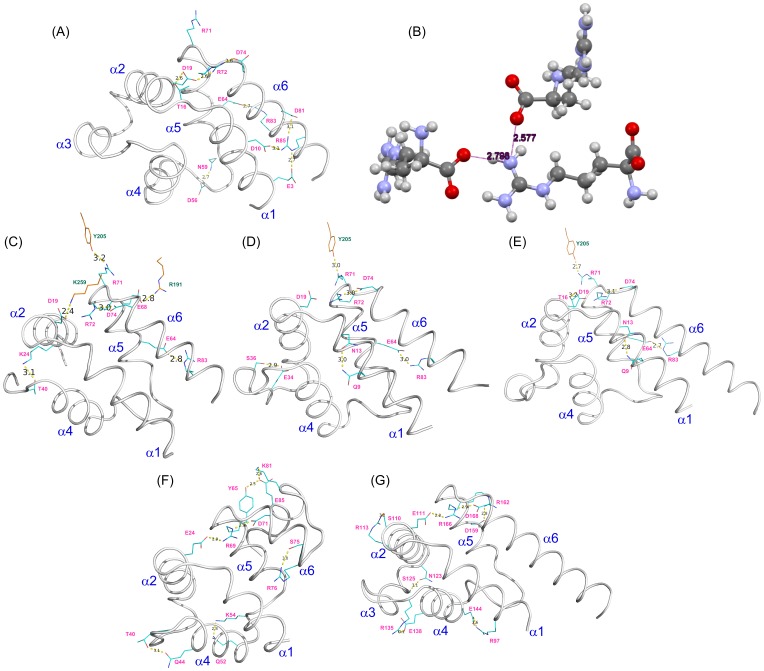
Figure 3. Electrostatic and hydrogen bonding interactions between charged and polar amino acid side chains.
(A) PEA-15 DED in the free form (PDB ID 2LS7). (B) L-arginine (CSD Refcode TAQBIY). (C) Full-length PEA-15 complexed with T185E ERK2 (PDB ID 4IZ5). (D) PEA-15 DED complexed with unphosphorylated ERK2 (PDB ID 4IZ7). (E) PEA-15 DED complexed with dual phosphorylated ERK2 (PDB ID 4IZA). (F) MC159 protein DED1 (PDB ID 2BBR). (G) MC159 protein DED2 (PDB ID 2BBR). The six helices, α1–α6, are labeled on each DED structure, with residues forming hydrogen bonds represented as a stick model (cyan color with pink labels), and heavy atom distances (in Å) shown between the two residues. Residues from ERK2 (C–E) are colored in orange with green labels.