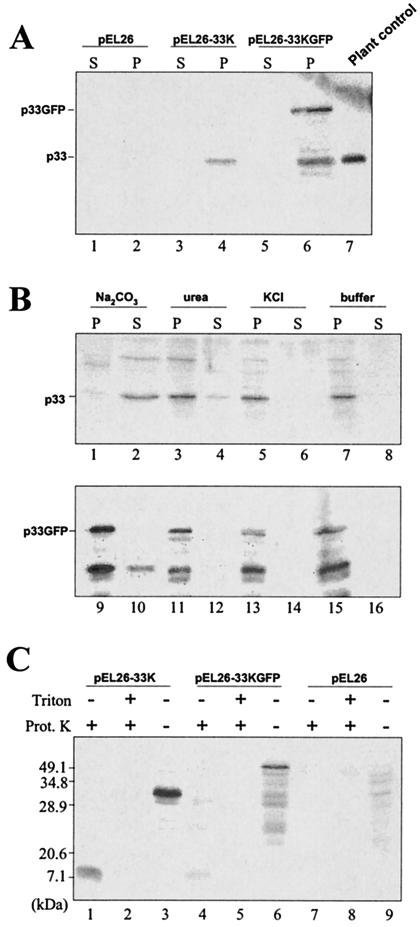
FIG. 3.
Western blot analysis of protein extracts from pEL26-GFP, pEL26-33K, and pEL26-33KGFP yeast cells fractionated in a 30,000 × g pellet (P) and supernatant (S). (A) Accumulation of nonfused and GFP-fused p33 in the pellet. The sizes of the protein expressed in yeast were determined by comparison with p33 extracted from a CymRSV-infected control plant (lane 7). Note the occurrence of both p33 and p33GFP in the same extract (lane 6). (B) Immunoblot analysis of membrane extracts from cells expressing p33 (upper blot) or p33GFP (lower blot) treated with carbonate, urea, or KCl and then separated by centrifugation into supernatant and pellet fractions. The resistance to carbonate extraction was weak with unfused p33 and higher with fused p33GFP. Conversely, resistance to urea and high-salt extraction was compatible with the properties of integral proteins. (C) Protein extracts from pEL26-33K, pEL26-33KGFP, and pEL26 transformed yeasts were treated with proteinase K in the presence or absence of Triton X-100. A protected fragment of equal size was obtained with unfused and fused p33.