Figure 1.
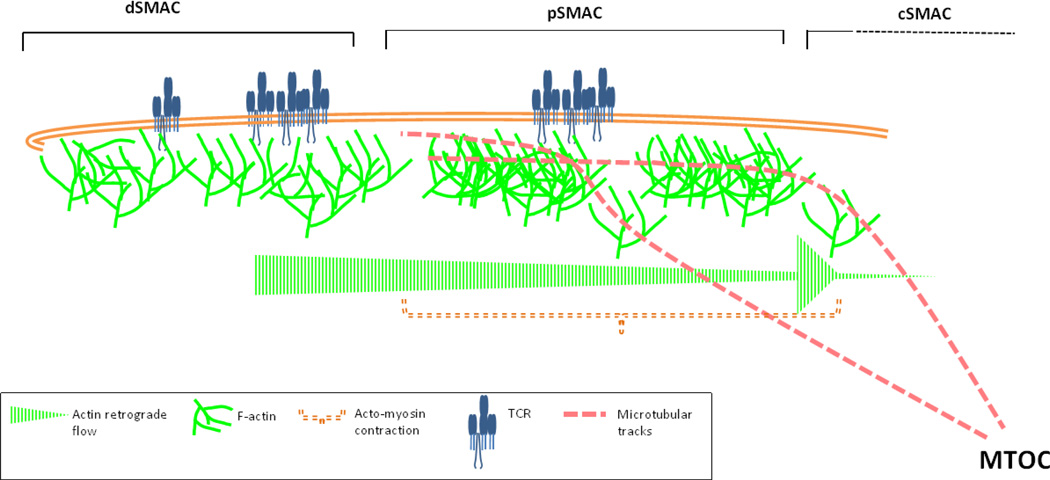
Actin polymerization and remodeling at the synapse is critical for TCR microcluster movement to the cSMAC. The mature radially symmetrical synapse exhibits three spatially distinct zones of actin organization. The outermost ring of rapid polymerization zone - dSMAC-involves feedback between TCR triggering and actin dynamics. Actin polymerization aids in TCR signaling and microcluster formation via an unknown mechanism, and TCR triggering in turn potentiates actin polymerization. Actin polymerization then drives movement of TCR microclusters from the dSMAC, towards the pSMAC, and is the onset site of fast actin centripetal flow (0.1µm/sec). The pSMAC is the middle zone that undergoes contractions guided by actomyosin network, and actin retrograde flow. Actin flow occurs at slower speed in the pSMAC (0.04µm/sec), and is achieved majorly via actin polymerization and possibly with contribution from myosinII activity. The contractile network is visible as F-actin “arcs” in Jurkat T cell system (shown as interrupted network in pSMAC). TCR microclusters exhibit kinetic coupling with actin flow all across the synapse interface; sometimes translocating slower than the flow due to occasional slippage, and sometimes exhibiting similar translocation rate as F-actin speckles. At the inner boundary of the pSMAC, there is rapid decline and collapse of F-actin flow, generating a sharp boundary of the F-actin-rich pSMAC and F-actin-poor cSMAC. Actin polymerization appears to be a major driving force for microcluster movement, however the myosinII activity and actomyosin contraction provides directional persistence to reduce meandering and efficient delivery of microclusters to the cSMAC. In addition, TCR microclusters are capable of interacting with microtubular motor protein dynein, thus utilizing microtubular tracks for navigating the synaptic interface, and eventual delivery to the cSMAC.