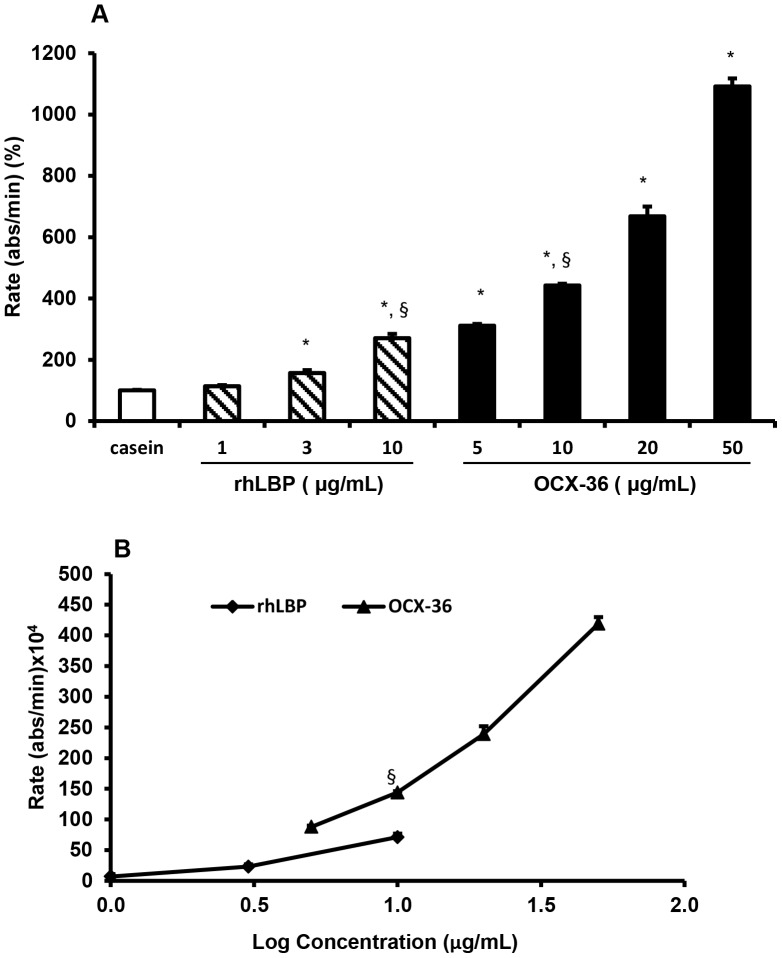
Figure 8. LPS binding activity of purified OCX-36 and rhLBP towards biotinylated E.coli O111:B4 LPS.
The LPS binding activity of OCX-36 purified from standard eggs (5, 10, 20 and 50 µg/mL) and recombinant human Lipopolysaccharide binding protein (rhLBP) (1, 3 and 10 µg/mL) was tested using the biotinylated LPS plate-binding assay. (A) The LPS binding activity of rhLBP and OCX-36 were significantly different than the negative control, casein (* p<0.05). (B) OCX-36 and rhLBP proteins showed a significant binding towards biotinylated E. coli O111:B4 LPS and OCX-36 was significantly higher than the positive control, rhLBP at 10 µg/mL (§ p<0.05). The LPS binding activity of both protein were normalized to casein. The results are three individual experiments and each experiment was performed in triplicate.