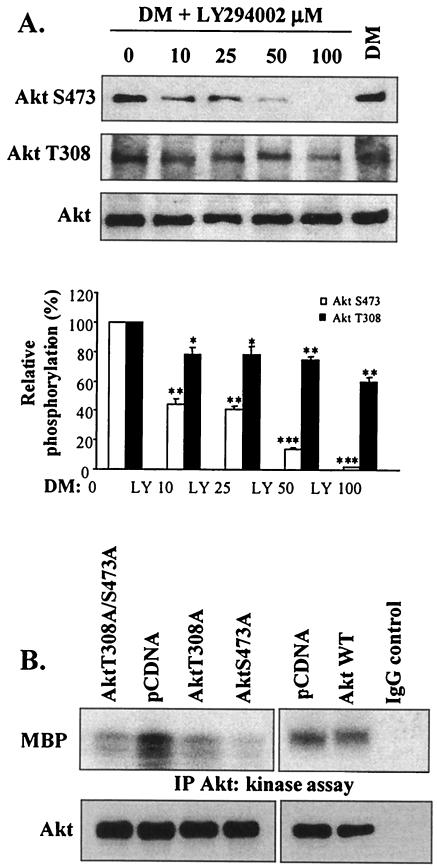
FIG. 3.
Phosphorylation of both sites in Akt (which are differentially regulated by PI 3-kinase) is essential in myogenesis. (A) Western blot analysis of Akt phosphorylation and total Akt levels in the presence of increasing concentrations of LY294002 after 72 h in DM. As a control, cells were treated with vehicle alone (DMSO; first lane). Quantification (by scanning densitometry) of the immunoblots was performed; data are expressed as percentages of Akt phosphorylation above control sample results. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared with DMSO results). (B) Akt kinase activity (after 72 h in DM) in lysates of C2 cells transiently transfected with AktT308A or AktS473A; as controls, C2 cells were also transfected with either empty vector (pCDNA) or the dominant-negative Akt double mutant AktT308A/S473A or wild-type Akt1 (Akt1 WT). As a control, kinase activity was examined using non-Akt binding IgG substituted for the anti-Akt antibody; assay Akt levels were determined by Western blotting with anti-Akt antibody (lower panels). IP, immunoprecipitation.