FIG. 6.
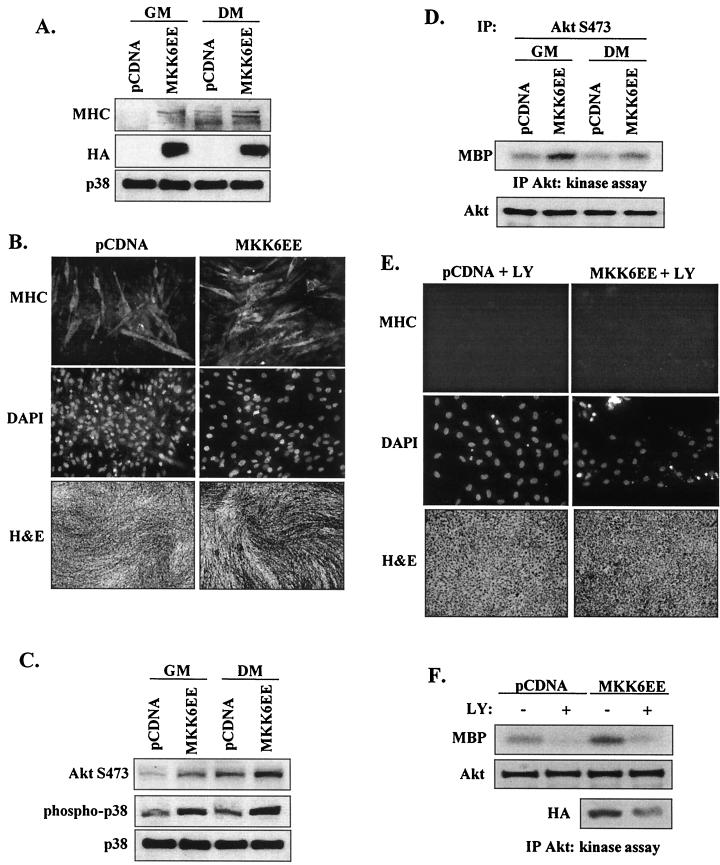
Activation of Akt by p38 MAPK requires PI 3-kinase activity. (A) Constitutively active MKK6EE stimulates myoblast differentiation of C2 cells. Proliferative (GM) and differentiating (DM) myoblasts were transfected with MKK6EE or control vector (pCDNA); after 48 h, expression of MHC was determined by Western blotting. The results of expression of HA-tagged MKK6EE in the cell lysates and total p38 levels are shown. (B) Myoblast differentiation in cells transfected with MKK6EE was assessed after 48 h in DM by immunofluorescence (×20 magnification) with anti-MHC antibody (upper panels), cell nuclei were visualized by DAPI staining (middle panels), and cell morphology was examined by H and E staining (×5 magnification) (lower panels). (C) Phosphorylation of AktS473 and p38 in proliferative (GM) and differentiating (DM) myoblasts transfected with MKK6EE or control vector (pCDNA) was determined by Western blotting after 48 h. Total p38 levels were measured to confirm even loading. (D) Akt kinase activity in proliferative (GM) and differentiating (DM) myoblasts transfected with MKK6EE or pCDNA after 48 h. C2 cells were harvested, and lysates were immunoprecipitated (IP) with an AktS473-phosphospecific antibody; total Akt levels were determined by Western blotting with anti-Akt antibody (lower panel). (E) Myoblast differentiation and morphology in cells transfected with MKK6EE or pCDNA in the presence or absence of LY294002 (10 μM), as assessed by immunofluorescence with anti-MHC antibody and by H and E staining. (F) Akt kinase activity in cells lysates from myoblasts transfected with MKK6EE or pCDNA in the presence (+) or absence (−) of LY and incubated in DM for 48 h. Total Akt and HA-tagged MKK6EE levels were determined by Western blotting (lower two panels).