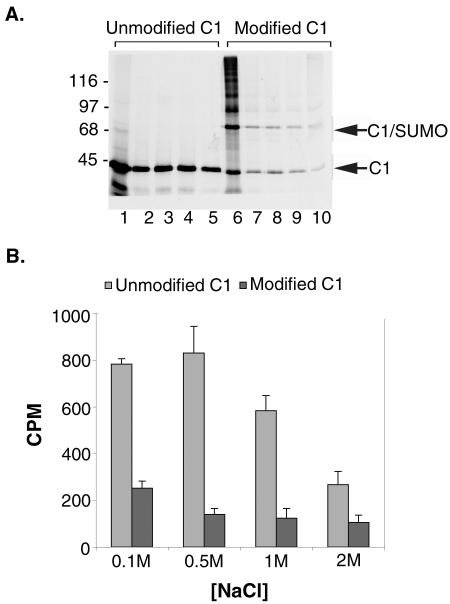
FIG. 7.
SUMO modification influences the nucleic acid binding activity of the hnRNP C proteins. hnRNP C1 was translated in rabbit reticulocyte lysate in the presence of [35S]methionine and incubated with ssDNA-cellulose before or following modification by SUMO-1. Equal numbers of radioactive protein counts were added to all binding reactions. Binding reactions were performed in buffers containing 0.1, 0.5, 1, and 2 M NaCl. (A) Proteins bound to the ssDNA-cellulose beads were eluted with SDS sample buffer and analyzed by SDS-PAGE followed by autoradiography. Molecular mass markers are indicated on the left, and unmodified and SUMO-modified hnRNP C1 proteins are indicated on the right. (B) Proteins bound to the ssDNA-cellulose beads were eluted with SDS sample buffer, and binding was quantified by determining bound radioactive counts using a scintillation counter. Binding reactions were carried out in triplicate, and average values and standard deviations were plotted with bound counts per minute (CPM) on the y axis and salt concentrations on the x axis.