Abstract
Background and Objectives:
High fructose corn syrup (HFCS) is the most commonly used sweetener in the United States. Some studies show that HFCS consumption correlates with obesity and insulin resistance, while other studies are in disagreement. Owing to conflicting and insufficient scientific evidence, the safety of HFCS consumption remains controversial.
Subjects/Methods:
We investigated the metabolic consequences of mice fed a (a) regular diet, (b) ‘Western' high-fat diet or (c) regular diet supplemented with 8% HFCS in drinking water (to mimic soft drinks) for 10 months. Adipose tissue macrophages (ATMs) have emerged as a major pathogenic factor for obesity and insulin resistance. ATMs consist of proinflammatory F4/80+CD11c+ macrophages and anti-inflammatory F4/80+CD11c− macrophages. In this study, we assessed the effects of HFCS on ATMs in intra-abdominal fat.
Results:
We found that HFCS feeding in mice induced more severe adipose inflammation and insulin resistance than even the higher-calorie-containing ‘Western' high-fat diet, and these HFCS-induced deleterious effects were independent of calorie intake or body fat content. We showed that similar to ‘Western' high-fat diet, HFCS triggered a robust increase of both proinflammatory ATMs and anti-inflammatory ATMs in intra-abdominal fat. Remarkably, however, the anti-inflammatory ATMs were much less abundant in HFCS-fed mice than in high-fat-fed mice. Furthermore, we showed that deletion of the ghrelin receptor (growth hormone secretagogue receptor, GHS-R) ameliorates HFCS-induced adipose inflammation and insulin resistance. HFCS-fed GHS-R-null mice exhibit decreased proinflammatory ATMs in intra-abdominal fat, reduced adipose inflammation and attenuated liver steatosis.
Conclusion:
Our studies demonstrate that HFCS has detrimental effects on metabolism, suggesting that dietary guidelines on HFCS consumption for Americans may need to be revisited. GHS-R deletion mitigates the effects of HFCS on adipose inflammation and insulin resistance, suggesting that GHS-R antagonists may represent a novel therapy for insulin resistance.
Keywords: ghrelin receptor, HFCS, macrophages, inflammation, insulin resistance, liver steatosis
Introduction
Obesity has become an epidemic in the United States; obesity-associated type 2 diabetes and metabolic syndrome are rising at an alarming rate.1 Since the 1970s, high fructose corn syrup (HFCS) has become ubiquitous in food supplies in the United States, substantially replacing sucrose (table sugar). HFCS-55 (55% fructose and 45% glucose) is the most commonly used form of HFCS in soft drinks. Studies have suggested that increased consumption of HFCS- or fructose-containing drink is linked to obesity and/or insulin resistance.2, 3, 4, 5, 6, 7 It was reported that fructose-sweetened beverages (but not glucose-sweetened beverages) increase visceral adiposity and insulin resistance in humans.6 A study comparing the short-term metabolic and endocrine effects of beverages sweetened with glucose, fructose, HFCS and sucrose found that fructose-containing beverages increase postprandial triglycerides more than glucose-sweetened beverages.7 This result suggests that fructose, regardless of the form in which the sweetener is used, promotes obesity in humans. However, other reports support a conclusion that calories from HFCS are no different from those of sucrose.8, 9 It is important to note that most of the studies were carried out using ‘normal' dietary fructose, while very few studies utilized HFCS. One report in rats showed that 8% HFCS-55 in drinking water promoted weight gain and increased abdominal fat; however, adipose inflammation and insulin sensitivity were not examined.10 Also, most published studies are based on short-term treatment, and the amounts of fructose or HFCS used were hugely variable, which may have contributed to the variable outcomes.6, 7, 11, 12 Since the scientific evidence on whether HFCS is involved in the pathogenesis of obesity and insulin resistance is inconclusive, HFCS continues to be used as the primary sweetener in our food supplies.
Ghrelin is the only known circulating orexigenic hormone. Ghrelin signaling has increasingly been recognized as a key regulator of obesity, insulin resistance and diabetes;13, 14 intriguingly, recent evidence shows that many of these regulatory functions appear to be independent of ghrelin's effect on food intake.15, 16, 17, 18 Growth hormone secretagogue receptor (GHS-R) is considered a biologically relevant receptor for ghrelin.13, 14 While dietary glucose suppresses postprandial ghrelin secretion, dietary fructose and fat have much less suppressive effects on postprandial ghrelin secretion.19, 20 High-fat diet promotes a state of chronic low-grade inflammation in adipose tissues, which leads to insulin resistance.21, 22, 23 Adipose tissue macrophages (ATMs) have been shown to have important roles in obesity, adipose inflammation and insulin resistance.24, 25, 26 ATMs release inflammatory cytokines, which impair adipocyte function by inhibiting insulin action. Diet-induced obesity has been shown to be associated with a phenotypic switch from anti-inflammatory ATMs (F4/80+CD11c−) to proinflammatory ATMs (F4/80+CD11c+), perpetuating an inflammatory state.24, 25 ATMs are specialized macrophages; their functional characteristics are dependent on the surrounding microenvironment of adipocytes.27 Both ghrelin and GHS-R are expressed in lymphocytes and macrophages,28, 29, 30 but it is unknown whether ghrelin/GHS-R signaling affects ATMs. Ghrelin has been shown to have anti-inflammatory effect in T cells and macrophages,28, 29, 30, 31, 32, 33 but it is unknown whether the effect of ghrelin is mediated through GHS-R. We and others have shown that ghrelin induces GH release, food intake and abdominal obesity via GHS-R-dependent mechanisms;14, 34 however, it regulates thermogenesis, sleep, osteoblast growth and liver glucose output by mechanisms independent of GHS-R.35, 36, 37, 38 Thus, it is important to investigate the direct effect of GHS-R in ATMs to fully understand the roles of ghrelin and GHS-R in adipose inflammation.
In this study, we compared the long-term metabolic effects of (10 months) regular diet (RD), ‘Western' high-fat/high sucrose diet (HFD) and RD supplemented with ‘soft drink' of 8% HFCS-55 (HFCS) in mice. We found that among the three feeding regimens, HFCS-fed mice exhibited the most severe insulin resistance. The HFCS has deleterious effects on insulin sensitivity, which were disproportionate to caloric intake or total body fat content. Our flow cytometry studies suggest that similar to HFD, HFCS induces robust increases of both proinflammatory ATMs (F4/80+CD11c+) and anti-inflammatory ATMs (F4/80+CD11c−) in intra-abdominal fat. However, anti-inflammatory ATMs were much less abundant in HFCS-fed mice than in HFD-fed mice. Moreover, our data suggest that GHS-R ablation (Ghsr−/− mice) attenuated HFCS-induced insulin resistance by reducing macrophage-mediated adipose inflammation and liver steatosis. Overall, our findings indicate that HFCS consumption has detrimental effects on insulin sensitivity, and ablation of GHS-R attenuates HFCS-induced adipose inflammation and insulin resistance.
Materials and methods
Animals
The generation of Ghsr−/− mice has been described previously,14 and all mice were on a pure C57BL/6J background. Age-matched congenic male wild-type (WT) and Ghsr−/− mice were given ad libitum either an RD (RD 2920X: 6.5% fat, 60% carbohydrates, 19.1% protein calories; TD.2920X is an irradiated form of rodent diet 2020X; Harlan Teklad, Madison, WI, USA), a high-fat/high sucrose ‘Western' diet (HFD: 42% fat, 42.7% carbohydrates, 15.2% protein calories; TD.88137; Harlan Teklad) or an RD with 8% HFCS-55 that were similar to previously published results in rats10 (Formula 55, v v−1 dissolved in water; Nature's Flavors, Orange, CA, USA). All our experiments were approved by the IACUC committee of Baylor College of Medicine.
Metabolic parameters and quantitative real-time PCR
Body composition, indirect calorimetry, glucose tolerance test (GTT), insulin tolerance test (ITT) and real-time reverse transcription-polymerase chain reaction of the adipose tissues were performed as described previously.18, 35 Regular water replaced the HFCS-containing water during the fasting period before and during both GTTs and ITTs. A homeostasis model assessment of insulin resistance (HOMA-IR) was also used to evaluate insulin resistance, calculated with the following formula: fasting insulin (μU ml−1) × fasting plasma glucose (mmol l−1)/22.5.
Adipose tissue fractionation, macrophage isolation and flow cytometry analysis
Mouse epididymal adipose tissues were fractionated into adipocytes and stromal vascular fraction as described.39 Macrophages from the adipose tissues were isolated by magnetic beads, as described.40 Stromal vascular fraction cells from epididymal adipose tissues (1 × 106 in a volume of 100 μl of phosphate-buffered saline) were used for flow cytometry analysis as described.21, 22
Isolation of peritoneal macrophages and gene knockdown in macrophage cell culture
Mice were anesthetized and 5 ml of PBS containing 3% fetal calf serum was injected into the peritoneal cavity. After shaking gently for 2–3 min, peritoneal liquid was collected. Cells were spun down at 500 g for 5 min at 4 °C. The murine macrophage RAW264.7 cells were obtained from ATCC (TIB-71, Manassas, VA, USA), and cultured as described.41 The small interfering RNAs (siRNAs) were purchased from Sigma (St Louis, MO, USA): sense strand of siGHS-R, 5′-CCACAAACAGACAGUGAAGUU-3′ sense strand of scrambled RNA, 5′-CAACAACGAAGCGACAUAAUC-3′. GHS-R siRNA was transfected into RAW246.7 cells by Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instruction. Briefly, one million RAW264.7 cells were seeded in a 10 cm dish for 12 h. Two hours before transfection, the medium was changed. Ten nanomolar siRNA solution was mixed well with Lipofectamine 2000 reagent and kept at room temperature for 5 min. Then, siRNA mixture was added to the cell dish. After 12 h, cells were harvested for gene expression analysis.
Assessment of hepatic steatosis and statistical analyses
Hepatic steatosis was assessed using hematoxylin and eosin staining of paraffin sections and Oil-Red-O staining of frozen sections, according to routine immunohistochemistry protocols. We used repeated measures analysis of variance and two-tailed Student's t-test to determine statistical significance between genotypes or treatments.
Results
Effect of HFCS on energy homeostasis and insulin sensitivity
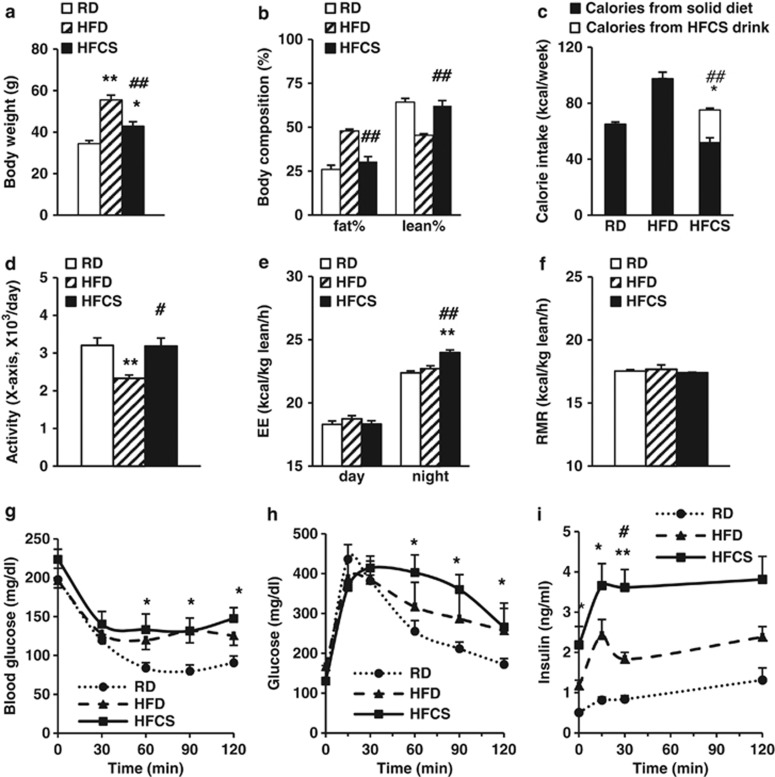
To evaluate the long-term effects of HFCS on energy homeostasis and metabolism, 2-month-old WT mice with similar body weights were randomly assigned to three groups and fed with RD, HFD or HFCS, respectively, for 10 months. The body weight of HFCS-fed mice was higher than that of RD-fed mice, but lower than that of HFD-fed mice (Figure 1a). HFCS-fed mice have similar fat and lean masses compared with RD-fed mice, but significantly lower fat mass than HFD-fed mice (Figure 1b). Total caloric intake of HFCS-fed mice was slightly higher than RD-fed mice, but much lower than HFD-fed mice (Figure 1c).
Figure 1.
The effects of different diets on metabolic parameters in WT mice. The WT mice were randomly assigned to one of three diets starting from 2 months of age: RD, HFD or regular chow with 8% HFCS in the drinking water. (a and b) Body weight and body composition analysis of the mice at 10 months of age. (c) Weekly caloric intake of the mice collected between 5 and 6 months of age. Calorie intake was calculated from both solid diet and HFCS drink. CLAMS was carried out when mice were 7 months old (after 5 months on respective diets). (d) Locomotor activity of the mice. (e and f) Average 24-h energy expenditure (EE) and resting metabolic rate (RMR) adjusted by lean mass. ITTs and GTTs were carried out at 8 months of age. (g) Glucose levels during ITT. (h and i) Glucose and insulin levels during GTT. n=8–10 in each group; *P<0.05, **P<0.001, RD vs HFCS or HFD; #P<0.05, ##P<0.001, HFCS vs HFD, respectively.
Indirect calorimetry analysis showed that the locomotor activity of HFCS-fed mice was similar to that of RD-fed mice, while HFD-fed mice showed reduced activity (Figure 1d). Interestingly, compared with RD- and HFD-fed mice, HFCS-fed mice had increased energy expenditure during the dark cycle, but not during the light cycle (Figure 1e). No significant differences were observed for resting metabolic rate among the three groups of mice (Figure 1f).
To determine whether HFCS-induced weight gain was linked to insulin sensitivity, ITTs and GTTs were performed. During ITTs, both HFD- and HFCS-fed mice demonstrated significantly reduced insulin responsiveness when compared with RD-fed mice (Figure 1g). During GTTs, HFCS-fed mice had lower glucose clearance rate compared with RD-fed mice (Figure 1h); remarkably, two- and four-fold higher plasma insulin levels were detected in HFCS-fed mice compared with HFD- and RD-fed mice, respectively (Figure 1i). In agreement with ITTs, HOMA-IR suggested that HFCS feeding is associated with severe insulin resistance: RD (6.47±0.23), HFD (19.04±2.30) and HFCS (32.7±14.72) (*P<0.05, HFCS or HFD vs RD). These data indicate that HFCS feeding causes more severe insulin resistance than even the higher-calorie HFD feeding.
Effect of HFCS on adipose inflammation
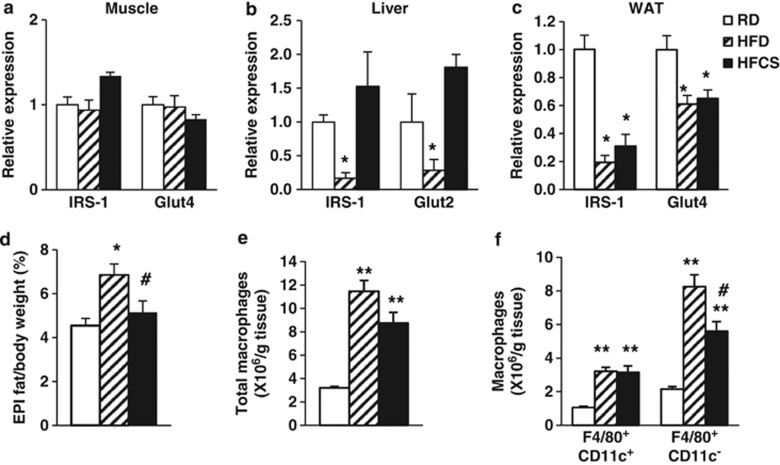
Diet-induced obesity impairs insulin action in many tissues, including skeletal muscle, liver and adipose tissues.42 Insulin receptor substrate 1 is a marker of insulin signaling, and glucose transporters (glucose transporter type 2 or 4 (Glut2 or Glut4)) are key mediators of glucose uptake in the tissues. Compared with RD feeding, HFCS feeding significantly reduced insulin receptor substrate 1 and Glut4 levels in epididymal white adipose tissue (WAT), but not in the muscle or liver (Figures 2a–c). This suggests that WAT has an important role in HFCS-induced insulin resistance; we thus primarily focused our studies on adipose tissues. We weighed epididymal fat depots from mice fed with three different diets. Compared with RD feeding, HFD feeding increased the ratio of epididymal fat:body weight, but HFCS feeding did not change the ratio significantly (Figure 2d).
Figure 2.
HFCS induces severe adipose tissue inflammation. Relative insulin receptor substrate 1 (IRS-1) and Glut4 expression in (a) skeletal muscle, (b) liver and (c) epididymal fat (EPI). (d) Percentage of EPI depot from the 12-month-old WT mice fed with either RD, HFD or HFCS for 10 months. Flow cytometry analysis of (e) total ATMs, (f) proinflammatory F4/80+CD11c+ and anti-inflammatory F4/80+CD11c− ATMs in the EPI. n=6–8; *P<0.05, **P<0.001, RD vs HFCS or HFD; #P<0.05, HFCS vs HFD, respectively.
Adipose tissue inflammation has an important role in insulin resistance; proinflammatory macrophage infiltration and proinflammatory cytokine release are the key mediators of inflammation in adipose tissues.21, 22, 23, 24 Using flow cytometry analysis, we found significant increase in total ATMs (F4/80+CD11c+ and F4/80+CD11c−) in both HFD- and HFCS-fed mice compared with RD-fed mice (Figure 2e). Although proinflammatory F4/80+CD11c+ ATMs were increased to the same degree after HFD and HFCS feeding, there were much less anti-inflammatory F4/80+CD11c−ATMs in HFCS-fed mice compared with HFD-fed mice (Figure 2f). This suggests that HFCS consumption promotes a more severe proinflammatory state in WAT, which is more pronounced than even the higher-calorie HFD feeding, and is consistent with the more severe insulin resistance observed in HFCS-fed mice.
Ablation of GHS-R attenuates HFCS-induced insulin resistance
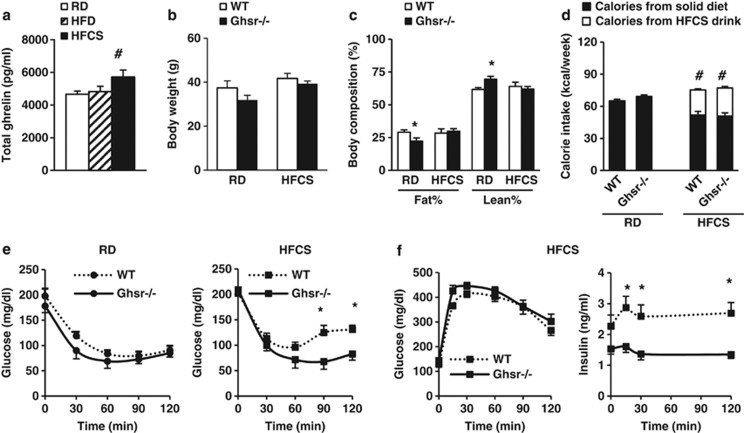
Dietary fructose attenuates the suppression of postprandial ghrelin.19 Indeed, our data showed that HFCS-fed mice had higher total ghrelin levels than that of RD- and HFD-fed mice (Figure 3a), which suggests that HFCS feeding may activate ghrelin signaling. Ghrelin stimulates appetite and promotes adiposity through its receptor GHS-R.14, 43 To further determine whether GHS-R mediates the effect of HFCS, age-matched congenic WT and Ghsr−/− mice were fed either RD or HFCS for 10 months, starting at 2 months of age. RD-fed Ghsr−/− mice had lower body fat compared with RD-fed WT mice, whereas HFCS-fed mice demonstrated no change in weight or body fat between the two genotypes (Figures 3b and c). HFCS-fed mice showed higher total calories intake compared with RD-fed mice, but no difference between genotypes feeding the same diet (Figure 3d).
Figure 3.
GHS-R ablation attenuates HFCS-induced insulin resistance independent of obesity. The WT and Ghsr−/− mice were fed either RD or HFCS starting from 2 months of age. (a) Total ghrelin levels of different diet-fed mice at 12 months of age. Mice were fasted overnight. (b and c) Body weight and body composition of the mice at 10 months of age. (d) Weekly calorie intake of the mice collected between 5 and 6 months of age. Calories were calculated from both solid diet and HFCS drink. ITTs and GTTs were carried out at 8 months of age. (e) Glucose levels during ITT. (f) Glucose and insulin levels of HFCS-fed mice during GTT. n=6–8; *P<0.05, **P<0.001, Ghsr−/− vs WT mice; #P<0.05, HFCS vs RD within WT or Ghsr−/− mice, respectively.
To evaluate the effect of GHS-R on insulin action under HFCS feeding, we performed ITTs and GTTs on the mice. ITTs showed no significant difference between RD-fed WT and Ghsr−/− mice. Interestingly, HFCS-fed Ghsr−/− mice had reduced glucose excursion during ITTs when compared with HFCS-fed WT mice, suggesting improved insulin sensitivity (Figure 3e). During GTTs, no difference was seen in glucose levels between HFCS-fed WT and Ghsr−/− mice. Remarkably, plasma insulin levels were significantly lower in HFCS-fed Ghsr−/− mice compared with HFCS-fed WT mice (Figure 3f), indicating that ablation of GHS-R mitigates HFCS-induced insulin resistance.
GHS-R ablation reduces adipose tissue inflammation
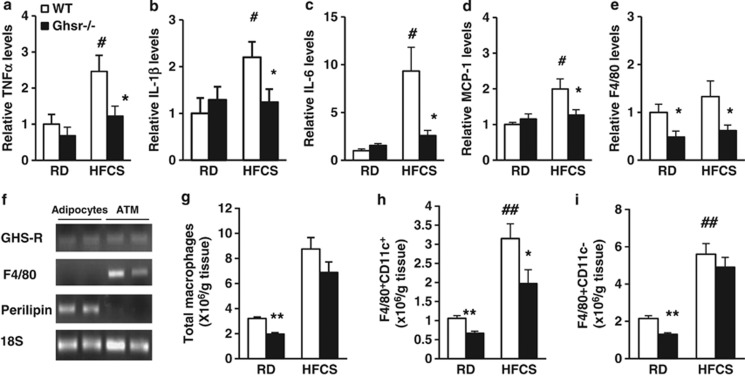
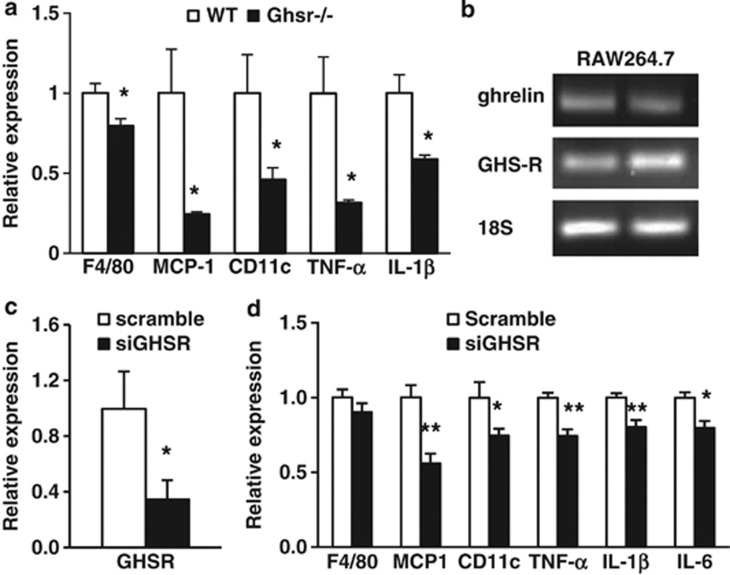
To investigate the effects of GHS-R ablation on HFCS-induced adipose inflammation, the mRNA expressions of proinflammatory cytokines (tumor necrosis factor-α (TNF-α), interelukin-1β (IL-1β) and IL-6), macrophage chemoattractant protein-1 (MCP-1) and macrophage marker F4/80 were evaluated in epididymal WAT. The expression levels of TNF-α, IL-1β, IL-6 and MCP-1 were significantly increased in WAT of HFCS-fed WT mice compared with RD-fed WT mice (Figures 4a–d). Similarly, HFCS-fed Ghsr−/− mice had lower expression levels of TNF-α, IL-1β, IL-6, MCP-1 and F4/80 in WAT. These results suggest that GHS-R is critical for HFCS-induced adipose inflammation (Figures 4a–e).
Figure 4.
Ablation of GHS-R improves HFCS-induced adipose inflammation. (a–e) mRNA expression of proinflammatory cytokines TNF-α, IL-1β and IL-6, and macrophage recruiting factor MCP-1 and macrophage marker F4/80 in epididymal fat of 12-month-old RD- and HFCS-fed WT and Ghsr−/− mice. (f) GHS-R is expressed in mature adipocytes and ATMs. (g) Flow cytometry analysis of total ATMs, (h) proinflammatory F4/80+CD11c+ and (i) anti-inflammatory F4/80+CD11c− ATMs in the epididymal fat of 12-month-old RD- and HFCS-fed WT and Ghsr−/− mice. n=5 in each group; *P<0.05, **P<0.001, Ghsr−/− vs WT mice under RD or HFCS; #P<0.05, ##P<0.001, RD vs HFCS in WT mice, respectively.
GHS-R is expressed in macrophages;28, 29, 30 however, its expression in ATMs has not been examined. To decipher whether the effects of GHS-R on adipose inflammation are mediated by adipocytes or ATMs, we isolated mature adipocytes and ATMs from epididymal fat of WT mice. The semiquantitative PCR results showed that GHS-R is expressed both in mature adipocytes and in ATMs (Figure 4f). Our flow cytometry studies further revealed that total ATMs were decreased in Ghsr−/− mice when compared with WT mice under RD feeding, but there was no difference under HFCS feeding (Figure 4g). However, Ghsr−/− mice showed reduced proinflammatory F4/80+CD11c+ ATMs under both RD and HFCS feeding, while the number of anti-inflammatory F4/80+CD11c− ATMs was only reduced under RD feeding but not under HFCS feeding (Figures 4h and i). Our results show decreased ratio of proinflammatory F4/80+CD11c+ vs anti-inflammatory F4/80+CD11c− in HFCS-fed Ghsr−/− mice, which supports reduced adipose inflammation.
Knockdown GHS-R suppresses inflammatory gene expression in macrophages
As GHS-R is expressed in adipocytes, macrophages, ATMs and other tissues, the inflammatory phenotype observed in adipose tissue of Ghsr−/− mice may be due to the effects of GHS-R on adipocytes and/or macrophages, or could be indirect. To assess the effect of GHS-R on macrophages, we isolated peritoneal macrophages from HFCS-fed Ghsr−/− and WT mice. The expression of F4/80 in peritoneal macrophages of Ghsr−/− mice was lower, suggesting that GHS-R ablation may reduce the total number of macrophages (Figure 5a). The expressions of MCP-1, proinflammatory cytokines (TNF-α and IL-1β) and proinflammatory macrophage marker CD11c in peritoneal macrophages were also significantly decreased in Ghsr−/− mice (Figure 5a). To further elucidate whether GHS-R has direct effect on macrophages, we studied lipopolysaccharide-induced inflammatory responses in a mouse macrophage cell line, RAW264.7 cells.30 Both GHS-R and ghrelin mRNAs were expressed in RAW264.7 cells (Figure 5b). GHS-R knockdown in RAW264.7 cells using GHS-R siRNA (knockdown efficiency was about 70% Figure 5c) decreased the expressions of MCP-1, proinflammatory macrophage marker (CD11c) and inflammatory cytokines (TNF-α, IL-1β and IL-6) when compared with scrambled RNA-treated cells (Figure 5d). F4/80 expression was not changed, indicating that knockdown in RAW264.7 cells did not alter macrophage characteristics. The data collectively suggest that GHS-R may directly regulate proinflammatory cytokine expression in macrophages and promote macrophage-mediated adipose inflammation.
Figure 5.
HFCS-fed Ghsr−/− mice have reduced inflammation in peritoneal macrophages, and GHS-R knockdown in macrophage RAW264.7 cells reduces inflammatory cytokine expression. (a) mRNA expression of F4/80, MCP-1, CD11c and proinflammatory cytokines (TNF-α and IL-1β) in peritoneal macrophages of HFCS-fed WT and Ghsr−/− mice. (b) Both ghrelin and GHS-R are expressed in RAW264.7 cells. (c) siRNA knockdown of GHS-R in RAW264.7 cells. (d) Lipopolysaccharide (LPS)-induced proinflammatory cytokine expression in GHS-R knockdown RAW264.7 cells. LPS (1 μg ml−1) was used overnight to induce inflammation. n=6; *P<0.05, **P<0.001, WT vs Ghsr−/− mice, or siGHSR vs Scramble, respectively.
Knockdown GHS-R attenuates HFCS-induced liver steatosis
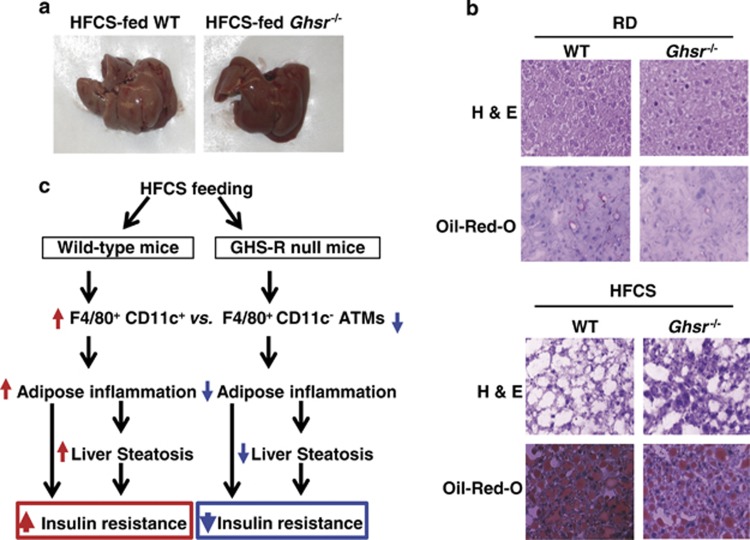
Adipose inflammation leads to liver steatosis.44 It was reported that fructose elicits harmful metabolic effects in the liver, including fatty liver and inflammation.45, 46, 47 Therefore, we performed morphological and histological analyses on the livers. Livers of HFCS-fed WT mice appeared pale, while livers of Ghsr−/− mice assumed normal-reddish appearance (Figure 6a). Hematoxylin and eosin and Oil Red-O staining further supported that HFCS feeding significantly increased lipid content in the liver and caused liver steatosis; the livers of HFCS-fed Ghsr−/− mice showed markedly reduced lipid accumulation and steatosis (Figure 6b). The data support that HFCS consumption induces liver steatosis, and GHS-R ablation attenuates it.
Figure 6.
Ablation of GHS-R attenuates HFCS-induced hepatic steatosis. (a) Pictures of the livers. (b) Hematoxylin and eosin staining and Oil-Red-O staining of the liver tissue of 12-month old RD- and HFCS-fed WT and Ghsr−/− mice. (c) Model proposed for the role of GHS-R in HFCS-mediated adipose inflammation and insulin resistance. In the WT mice, HFCS promotes proinflammatory F4/80+CD11c+ ATM infiltration, leading to adipose inflammation and liver steatosis, and thus resulting in insulin resistance. In the GHS-R null mice, GHS-R ablation decreases the ratio of proinflammatory F4/80+CD11c+ vs anti-inflammatory F4/80+CD11c−ATMs, leading to decreased adipose inflammation and reduced liver steatosis, and thus improving insulin sensitivity.
Discussion
Our results showed that HFCS feeding modestly increases body weight, total calorie intake and nighttime energy expenditure when compared with RD-fed mice, but showed no difference in body composition or activity (Figures 1a–f). Importantly, calorie intake of HFCS-fed mice was much lower than the calorie intake of HFD-fed mice (Figure 1c). Our data suggest that HFCS does not have significant effects on obesity (body fat), which is in agreement with the study of HFCS in lean women.9 Although fructose does not stimulate insulin secretion in the short-term feeding,48 long-term fructose feeding has been shown to induce compensatory hyperinsulinemia.21, 22, 23 In agreement, our ITT and GTT studies showed that HFCS consumption promotes severe whole-body insulin resistance (Figures 1g–i). HFCS-fed mice had glucose clearance similar to that of HFD-fed mice during GTT, but worse glucose clearance than RD-fed mice (Figure 1h). Remarkably, HFCS feeding was associated with significantly higher insulin than HFD feeding (Figure 1i). This indicates that HFCS is an even more potent inducer of insulin resistance than higher-calorie HFD, and that the detrimental effects of HFCS on insulin sensitivity are not simply because of the extra calories associated with HFCS. Thus, HFCS has distinctive properties, and calories from HFCS are even more harmful than those from HFD.
We have previously reported that ghrelin signaling regulates glucose-induced insulin secretion and insulin sensitivity.18 HFCS-fed mice revealed very pronounced insulin resistance (Figures 1g–i). The insulin resistance may be contributed by several key metabolic tissues, such as skeletal muscle, liver and WAT. Compared with RD feeding, HFCS feeding significantly reduced the expression of insulin signaling regulator insulin receptor substrate 1 and glucose transporter Glut4 in intra-abdominal adipose tissue, but not in the muscle or liver (Figures 2a–c). The data suggest that the HFCS-induced insulin impairment is probably mediated by adipose tissues. Interestingly, the ratio of epididymal fat:body weight of HFCS-fed mice was no different from that of RD-fed mice, while HFD feeding increased this ratio significantly (Figure 2d). The data are consistent with the body composition data (Figure 1b), suggesting that HFCS is less obesogenic than HFD.
Infiltration of macrophages into adipose tissues has been recognized as an important mechanism of insulin resistance.24, 25, 49 We found equal elevation of proinflammatory F4/80+CD11+ macrophages in epididymal fat of HFCS- and HFD-fed mice than RD-fed mice; however, the increase of anti-inflammatory F4/80+CD11c− macrophages was less prominent in HFCS-fed mice than in HFD-fed mice (Figures 2e and f). The data suggest that HFCS inflicts even more severe adipose inflammation than higher-calorie-containing HFD, which is in line with the robust insulin resistance exhibited by HFCS-fed mice (Figure 1i).
Both dietary fructose and glucose attenuate postprandial ghrelin secretion, but the suppression of postprandial ghrelin is much less pronounced after consuming fructose, as compared with glucose.19 Indeed, we detected higher total ghrelin with HFCS feeding than with RD or HFD feeding (Figure 3a), implying that HFCS may regulate ghrelin signaling differently from either RD or HFD diets. We and others have shown that ghrelin has GHS-R-dependent and -independent functions.35, 36, 38 To elucidate the role(s) of GHS-R in HFCS-induced obesity and insulin resistance, we studied the metabolic effects of RD and HFCS in WT and Ghsr−/− mice. Our data showed that ablation of GHS-R does not change body weight, body composition or caloric intake of HFCS-fed mice (Figures 3b–d). Interestingly, though, our functional studies showed that ablation of GHS-R improved whole-body insulin sensitivity under HFCS diet, and the effect was most pronounced after 90 min of insulin injection during ITT (Figures 3e and f). As there is no difference in body weight, body composition or food intake between HFCS-fed WT and Ghsr−/− mice, our data indicate that the effects of GHS-R on HFCS-induced insulin resistance is independent of orexigenic signaling.
HFCS induced higher TNF-α, IL-1β and IL-6 expression levels in adipose tissues, and the expression of these cytokines was suppressed in epididymal fat of HFCS-fed Ghsr−/− mice (Figures 4a–c), indicating that GHS-R deletion decreases HFCS-induced adipose inflammation. Moreover, we detected decreased MCP-1 and F4/80 in adipose tissue of HFCS-fed Ghsr−/− mice (Figures 4d and e), suggesting that GHS-R ablation probably attenuates HFCS-induced adipose inflammation by reducing ATMs. Thus, ablation of GHS-R reduces proinflammatory macrophage infiltration into adipose tissue, which may contribute to the improved adipose inflammation and whole-body insulin sensitivity exhibited in HFCS-fed Ghsr−/− mice.
The current study showed that GHS-R is expressed both in mature adipocytes and in adipose macrophages (Figure 4f). Macrophage infiltration into WAT has been shown to increase the secretion of proinflammatory cytokines and contributes to insulin resistance.24, 25, 49 Our flow cytometry data showed that HFCS increased both types of ATMs, but GHS-R ablation selectively attenuates proinflammatory F4/80+CD11c+ ATMs while having no effects on anti-inflammatory F4/80+CD11c− ATMs (Figures 4h and i). The ATM profiles of Ghsr−/− mice (Figures 4g–i) as well as the reduced adipose inflammation revealed in HFCS-fed Ghsr−/− mice (Figures 4a–e) are consistent with the insulin-sensitive phenotype exhibited by the null mice (Figures 3e and f). Taken together, the data suggest that ablation of GHS-R reduces HFCS-induced adipose tissue inflammation by reducing the infiltration of proinflammatory F4/80+CD11c+ macrophages. We believe that the effects of GHS-R in adipose tissue inflammation are primarily mediated through macrophages. However, we cannot totally exclude the involvement of adipocytes.
In HFCS-fed mice, GHS-R ablation reduces peritoneal macrophages as well as proinflammatory cytokine expression (Figure 5a). We showed that GHS-R is expressed both in mature adipocytes and in ATMs (Figure 4f). GHS-R may regulate the functions of adipose tissues by directly affecting adipocytes and/or macrophages. To determine whether GHS-R has direct effects on macrophages, we studied RAW264.7 cells. Both ghrelin and GHS-R were expressed in these cells (Figure 5b), and knockdown of GHS-R with siRNA suppressed the expression of proinflammatory cytokines (Figures 5c and d). The data suggest that the effect of GHS-R in ATMs is mediated, at least in part, through direct effect(s) of GHS-R in macrophages.
The liver is an important organ for regulating blood glucose through glycogenesis, glycogenolysis and gluconeogenesis. Fructose is primarily metabolized in the liver.50 It has been reported that fructose is a more potent stimulator of de novo hepatic lipogenesis than is glucose.51 It is also known that HFCS induces liver steatosis, which has a causative role in insulin resistance.45, 46, 47 Indeed, HFCS-fed mice exhibited significantly increased lipid content in the liver, which probably also contribute to the abnormality of insulin sensitivity (Figures 6a and b). Interestingly, GHS-R ablation attenuated HFCS-induced liver steatosis (Figures 6a and b). However, the effect of GHS-R in the liver is probably indirect, because we and others have not been able to detect appreciable GHS-R1a expression in the liver.38, 52, 53
Collectively, our findings show that: (1) HFCS promotes proinflammatory F4/80+CD11c+ macrophage infiltration in adipose tissues, leading to adipose inflammation and liver steatosis, which results in insulin resistance; (2) GHS-R ablation decreases the ratio of proinflammatory F4/80+CD11c+vs anti-inflammatory F4/80+CD11c− ATMs, leading to decreased adipose inflammation and reduced liver steatosis, thus improving insulin sensitivity (Figure 6c). Our data indicate that GHS-R has proinflammatory effects in ATMs, which is opposite from ghrelin's anti-inflammatory effect in ATMs. Thus, the functions of ghrelin and GHS-R in the immune system are complex; it is important to distinguish the effects of ghrelin and GHS-R in macrophages/ATMs.
In conclusion, our studies demonstrate for the first time that HFCS has a detrimental effect on adipose inflammation and insulin sensitivity, even more severe than higher-calorie-containing HFD. HFCS preferentially promotes proinflammatory macrophage recruitment into intra-abdominal fat, induces adipose inflammation and has additional deleterious effects on insulin sensitivity. This challenges the argument that ‘calories are calories', and demonstrates that the metabolic perturbations caused by HFCS are above and beyond the extra calories associated with HFCS consumption. Immunometabolism is an emerging field of investigation in both immunology and metabolism.54 Our new findings suggest that HFCS may regulate metabolism by immunometabolism, but may not by obesity per se. GHS-R ablation attenuates HFCS-induced adipose inflammation and insulin resistance, suggesting that GHS-R is an important regulator mediating HFCS-induced adipose inflammation and insulin resistance. GHS-R may thus represent a novel mechanism linking nutrient sensing to immunometabolism. GHS-R antagonists may have the potential to be a new therapeutic option for treating insulin resistance by selectively targeting proinflammatory macrophages in adipose tissues, and thus attenuating diet-induced insulin resistance.
Acknowledgments
Measurements of body composition, energy balance and food intake were performed in the Mouse Metabolic Research Unit at the USDA/ARS Children's Nutrition Research Center (CNRC), Baylor College of Medicine, which is supported by funds from the USDA ARS (http: //www.bcm.edu/cnrc/mmru/). We acknowledge the expert assistance of Mr Firoz Vohra and the MMRU Core Director, Dr Marta Fiorotto. We also thank Drs Morey W Haymond, Douglas Burrin and Alli M Antar at CNRC of Baylor College of Medicine for their insightful input into this project, and Mr Michael R Honig at Houston's Community Public Radio Station KPFT for his editorial assistance. This work is a publication of the United States Department of Agriculture (USDA/ARS) Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, and has been funded in part with federal funds from the USDA/ARS under Cooperative Agreement No. 58-6250-0-008 (YS). This work was also supported by: 1R03AG029641-0 (YS), American Heart Association (AHA) innovative grant 12IRG9230004 (YS), the NIH-Diabetes and Endocrinology Research Center grant at Baylor College of Medicine P30DK079638 (YS), NIH/NIDDK grant R01DK080306 (DSH), Mouse Metabolic Phenotyping Center at Vanderbilt University (DK59637) and Animal Physiology Core at The Methodist Hospital Research Institute (directed by Dr Willa A Hsueh).
The authors declare no conflict of interest.
References
- Narayan KM, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on lifetime risk for diabetes in the US. Diabetes Care. 2007;30:1562–1566. doi: 10.2337/dc06-2544. [DOI] [PubMed] [Google Scholar]
- Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- Lustig RH. Childhood obesity: behavioral aberration or biochemical drive? Reinterpreting the First Law of Thermodynamics. Nat Clin Pract Endocrinol Metab. 2006;2:447–458. doi: 10.1038/ncpendmet0220. [DOI] [PubMed] [Google Scholar]
- Mietus-Snyder ML, Lustig RH. Childhood obesity: adrift in the ‘limbic triangle'. Annu Rev Med. 2008;59:147–162. doi: 10.1146/annurev.med.59.103106.105628. [DOI] [PubMed] [Google Scholar]
- Ishimoto T, Lanaspa MA, Le MT, Garcia GE, Diggle CP, Maclean PS, et al. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc Natl Acad Sci USA. 2012;109:4320–4325. doi: 10.1073/pnas.1119908109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhope KL, Havel PJ. Fructose consumption: considerations for future research on its effects on adipose distribution, lipid metabolism, and insulin sensitivity in humans. J Nutr. 2009;139:1236S–1241S. doi: 10.3945/jn.109.106641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup. Am J Clin Nutr. 2008;88:1733S–1737S. doi: 10.3945/ajcn.2008.25825D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melanson KJ, Angelopoulos TJ, Nguyen V, Zukley L, Lowndes J, Rippe JM. High-fructose corn syrup, energy intake, and appetite regulation. Am J Clin Nutr. 2008;88:1738S–1744SS. doi: 10.3945/ajcn.2008.25825E. [DOI] [PubMed] [Google Scholar]
- Melanson KJ, Zukley L, Lowndes J, Nguyen V, Angelopoulos TJ, Rippe JM. Effects of high-fructose corn syrup and sucrose consumption on circulating glucose, insulin, leptin, and ghrelin and on appetite in normal-weight women. Nutrition. 2007;23:103–112. doi: 10.1016/j.nut.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Bocarsly ME, Powell ES, Avena NM, Hoebel BG. High-fructose corn syrup causes characteristics of obesity in rats: increased body weight, body fat and triglyceride levels. Pharmacol Biochem Behav. 2010;97:101–106. doi: 10.1016/j.pbb.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76:911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- Stanhope KL. Role of fructose-containing sugars in the epidemics of obesity and metabolic syndrome. Annu Rev Med. 2012;63:329–343. doi: 10.1146/annurev-med-042010-113026. [DOI] [PubMed] [Google Scholar]
- Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA. 2004;101:4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, et al. UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Tilve D, Heppner K, Kirchner H, Lockie SH, Woods SC, Smiley DL, et al. Ghrelin-induced adiposity is independent of orexigenic effects. FASEB J. 2011;25:2814–2822. doi: 10.1096/fj.11-183632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 2006;3:379–386. doi: 10.1016/j.cmet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Lin L, Saha PK, Ma X, Henshaw IO, Shao L, Chang BH, et al. Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues. Aging Cell. 2011;10:996–1010. doi: 10.1111/j.1474-9726.2011.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teff KL, Elliott SS, Tschop M, Kieffer TJ, Rader D, Heiman M, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–2972. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, Gaylinn BD, et al. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab. 2008;93:1971–1979. doi: 10.1210/jc.2007-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake DK, Smith EO, Mersmann H, Smith CW, Robker RL. ICAM-1 expression in adipose tissue: effects of diet-induced obesity in mice. Am J Physiol Cell Physiol. 2006;291:C1232–C1239. doi: 10.1152/ajpcell.00008.2006. [DOI] [PubMed] [Google Scholar]
- Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Antunes C, Geliang G, Liu ZW, Borok E, Nie Y, et al. Agrp neurons mediate Sirt1's action on the melanocortin system and energy balance: roles for Sirt1 in neuronal firing and synaptic plasticity. J Neurosci. 2010;30:11815–11825. doi: 10.1523/JNEUROSCI.2234-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58:2574–2582. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H, et al. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125:2892–2903. doi: 10.1161/CIRCULATIONAHA.111.087817. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, et al. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109:2221–2226. doi: 10.1161/01.CIR.0000127956.43874.F2. [DOI] [PubMed] [Google Scholar]
- Waseem T, Duxbury M, Ito H, Ashley SW, Robinson MK. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery. 2008;143:334–342. doi: 10.1016/j.surg.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hataya Y, Akamizu T, Hosoda H, Kanamoto N, Moriyama K, Kangawa K, et al. Alterations of plasma ghrelin levels in rats with lipopolysaccharide-induced wasting syndrome and effects of ghrelin treatment on the syndrome. Endocrinology. 2003;144:5365–5371. doi: 10.1210/en.2003-0427. [DOI] [PubMed] [Google Scholar]
- Demers A, Caron V, Rodrigue-Way A, Wahli W, Ong H, Tremblay A. A concerted kinase interplay identifies PPARgamma as a molecular target of ghrelin signaling in macrophages. PLoS One. 2009;4:e7728. doi: 10.1371/journal.pone.0007728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit VD. Adipose-immune interactions during obesity and caloric restriction: reciprocal mechanisms regulating immunity and health span. J Leukocyte Biol. 2008;84:882–892. doi: 10.1189/jlb.0108028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JS, Kotokorpi P, Eccles SR, Barnes SK, Tokarczuk PF, Allen SK, et al. Ghrelin induces abdominal obesity via GHS-R-dependent lipid retention. Mol Endocrinol. 2009;23:914–924. doi: 10.1210/me.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Lin L, Qin G, Lu X, Fiorotto M, Dixit VD, et al. Ablations of ghrelin and ghrelin receptor exhibit differential metabolic phenotypes and thermogenic capacity during aging. PLoS One. 2011;6:e16391. doi: 10.1371/journal.pone.0016391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai E, Kapas L, Sun Y, Smith RG, Krueger JM. The preproghrelin gene is required for the normal integration of thermoregulation and sleep in mice. Proc Natl Acad Sci USA. 2009;106:14069–14074. doi: 10.1073/pnas.0903090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhanty PJ, van der Eerden BC, van der Velde M, Gauna C, Pols HA, Jahr H, et al. Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. J Endocrinol. 2006;188:37–47. doi: 10.1677/joe.1.06404. [DOI] [PubMed] [Google Scholar]
- Gauna C, Delhanty PJ, Hofland LJ, Janssen JA, Broglio F, Ross RJ, et al. Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes. J Clin Endocrinol Metab. 2005;90:1055–1060. doi: 10.1210/jc.2004-1069. [DOI] [PubMed] [Google Scholar]
- Vasudevan AR, Wu H, Xydakis AM, Jones PH, Smith EO, Sweeney JF, et al. Eotaxin and obesity. J Clin Endocrinol Metab. 2006;91:256–261. doi: 10.1210/jc.2005-1280. [DOI] [PubMed] [Google Scholar]
- Metwali A, Blum AM, Elliott DE, Weinstock JV. IL-4 inhibits vasoactive intestinal peptide production by macrophages. Am J Physiol Gastrointest Liver Physiol. 2002;283:G115–G121. doi: 10.1152/ajpgi.00491.2001. [DOI] [PubMed] [Google Scholar]
- Fraser I, Liu W, Rebres R, Roach T, Zavzavadjian J, Santat L, et al. The use of RNA interference to analyze protein phosphatase function in mammalian cells. Methods Mol Biol. 2007;365:261–286. doi: 10.1385/1-59745-267-X:261. [DOI] [PubMed] [Google Scholar]
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- Longo KA, Charoenthongtrakul S, Giuliana DJ, Govek EK, McDonagh T, Qi Y, et al. Improved insulin sensitivity and metabolic flexibility in ghrelin receptor knockout mice. Regul Pept. 2008;150:55–61. doi: 10.1016/j.regpep.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol. 2008;295:G987–G995. doi: 10.1152/ajpgi.90272.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison KS, Saleh SM, Bakheet RH, Al-Rabiah RK, Inglis AL, Makhoul NJ, et al. Diabetes of the liver: the link between nonalcoholic fatty liver disease and HFCS-55. Obesity (Silver Spring, MD) 2009;17:2003–2013. doi: 10.1038/oby.2009.58. [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Perez-Pozo SE, Sautin YY, Manitius J, Sanchez-Lozada LG, Feig DI, et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes. Endocr Rev. 2009;30:96–116. doi: 10.1210/er.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry DL. Effects of mannose and fructose on the synthesis and secretion of insulin. Pancreas. 1989;4:2–9. doi: 10.1097/00006676-198902000-00002. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basciano H, Federico L, Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab (Lond) 2005;2:5. doi: 10.1186/1743-7075-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerstein MK, Schwarz JM, Neese RA. Regulation of hepatic de novo lipogenesis in humans. Annu Rev Nutr. 1996;16:523–557. doi: 10.1146/annurev.nu.16.070196.002515. [DOI] [PubMed] [Google Scholar]
- Sun Y, Garcia JM, Smith RG. Ghrelin and growth hormone secretagogue receptor expression in mice during aging. Endocrinology. 2007;148:1323–1329. doi: 10.1210/en.2006-0782. [DOI] [PubMed] [Google Scholar]
- Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- Townsend K, Tseng YH. Brown adipose tissue: recent insights into development, metabolic function and therapeutic potential. Adipocyte. 2012;1:13–24. doi: 10.4161/adip.18951. [DOI] [PMC free article] [PubMed] [Google Scholar]