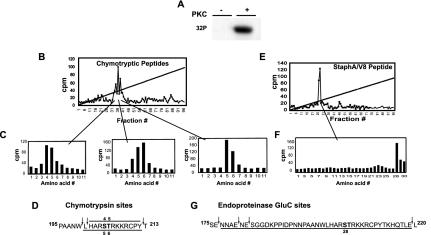
FIG. 3.
Identification of PKCα phosphorylation sites in the full-length FLAG-HOXA9 protein. (A) Affinity-purified His-tagged FLAG-HOXA9 was subjected to in vitro kinase assay with [γ-32]ATP in the absence or presence of purified PKC. (B) Chymotryptic peptides of PKCα-phosphorylated 32P-labeled FLAG-HOXA9, were separated by reverse-phase HPLC. (C) The radioactive fractions contributing to each peak were analyzed by Edman degradation. Sequencing of peak 1 released radioactivity in Edman cycles 4 and 5; similarly, sequencing of peaks 2 and 3 released radioactivity in cycles 4, 5, and 6 and cycles 5 and 6, respectively. (D) Sequence of the two overlapping FLAG-HOXA9 chymotryptic peptides, H201-Y212 and L200-Y212, which have serine and threonine residues in positions 4 and 5 and positions 5 and 6, respectively. Peak 1 was generated by chymotryptic peptide H201-Y212, peak 3 was generated by chymotryptic peptide L200-Y212, and peak 2 was generated by a mixture of the two overlapping chymotryptic peptides (E) Similarly, endoproteinase Glu-C peptides of 32P-labeled FLAG-HOXA9 were separated by reverse-phase HPLC and generated a single peak. (F) Edman sequencing of the endoproteinase Glu-C peak released the radioactivity in cycle 28. (G) Sequence of FLAG-HOXA9 with the predicted endoproteinase Glu-C cleavage sites, peptide N177-E219 has S204 at position 28.