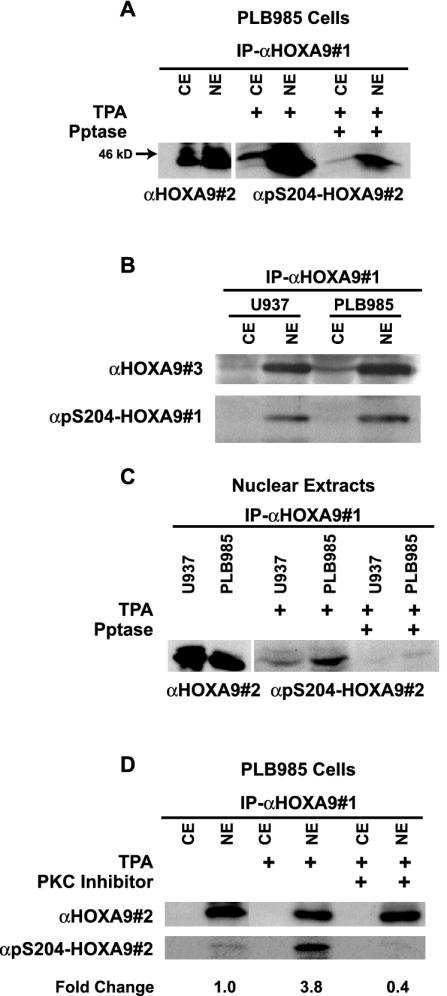
FIG. 5.
HOXA9 is phosphorylated at S204 in vivo and this phosphorylation is mediated by PKC. (A) Cytoplasmic or nuclear extracts of PLB985 cells were immunoprecipitated with goat α-HOXA9 antibody (αHOXA9#1). Western blot analysis of the immunoprecipitates using affinity-purified chicken α-HOXA9 antibody (αHOXA9#2) showed expression of HOXA9 predominantly in the nuclear extract and to a lesser extent in the cytoplasmic extract. HOXA9 immunoprecipitates from cytoplasmic and nuclear extracts of TPA-stimulated PLB985 cells, treated with or without phosphatase, were probed with α-pS204-HOXA9 antibody (#2). (B) HOXA9 protein from cytoplasmic and nuclear extracts from U937 and PLB985 cells treated with TPA was precipitated with αHOXA9#1 and detected with a rabbit affinity-purified α-HOXA9 antibody (#3) or a different α-pS204-HOXA9 antibody (#1). (C) Immunoprecipitates from nuclear extracts of U937 cells contained HOXA9 protein that was immunoreactive with αHOXA9#2 antibody. HOXA9 immunoprecipitates, from nuclear extracts of TPA-stimulated PLB985 and U937 cells, treated with or without phosphatase, were probed with α-pS204-HOXA9 antibody. (D) PLB985 cells stimulated with either TPA or control were pretreated with or without the PKC inhibitor bisindolylmaleamide1 (5 μM). Cytoplasmic and nuclear extracts were immunoprecipitated with αHOXA9#1, and analyzed by Western blotting with either αHOXA9#2 or α-pS204-HOXA9. Change in HOXA9 phosphorylation level was calculated by NIH ImageQuant analysis.