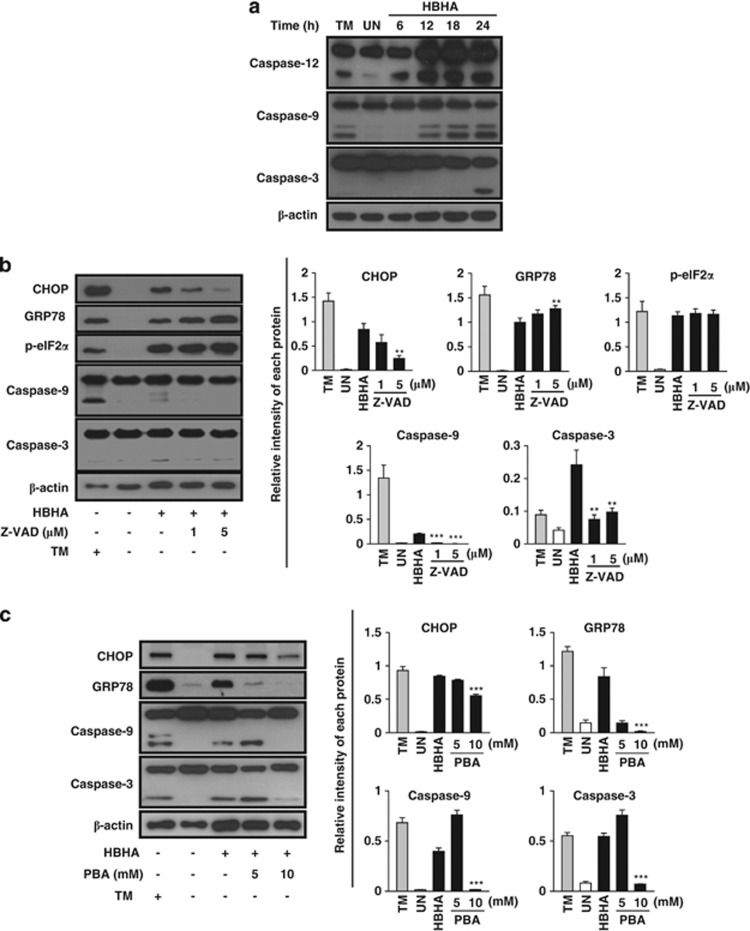
Figure 2.
The ER stress response is involved in HBHA-mediated and caspase-dependent apoptosis. (a) RAW 264.7 cells were stimulated with HBHA (10 μg/ml) for the indicated time period. The cell lysates were analysed by western blotting with antibodies for caspase-12, caspase-9, caspase-3, and β-actin. (b) RAW 264.7 cells were stimulated with HBHA for 24 h in the presence or absence of 1 h pretreatment with z-VAD-fmk. Next, total cell lysates were subjected to western blotting for CHOP, GRP78, p-eIF2α, caspase-9, caspase-3, and β-actin analysis. (c) RAW 264.7 cells were stimulated with HBHA for 24 h in the presence or absence of 1 h pretreatment with 4-PBA. Next, total cell lysates were subjected to western blotting for CHOP, GRP78, caspase-9, caspase-3, and β-actin analysis. A cell lysate treated with 2 μg/ml TM for 6 h was used as a positive control to induce ER stress. The blots were also probed for β-actin to control for loading. Bands corresponding to each protein were quantified, and the intensities of each protein were normalised to the intensity of actin. Data are representative of at least three independent experiments with similar results. The asterisks indicate significant differences compared with untreated cells (**P<0.01, ***P<0.001)