Abstract
Triatoma brasiliensis sensu lato (s.l.), the main vector of Chagas disease in northeastern Brazil, is a species complex comprising four species, one with two subspecies (T. brasiliensis brasiliensis, T. brasiliensis macromelasoma, T. juazeirensis, T. sherlocki, and T. melanica), and each taxon displaying distinct ecological requirements. In order to evaluate the genetic relationships among nine T. brasiliensis s.l. populations from northeastern Brazil, we analyzed their mitochondrial cytochrome c oxidase subunit 1 sequences and suggested a PCR-RFLP assay to distinguish between T. b. macromelasoma and T. b. brasiliensis subspecies. All the specimens were morphologically identified as T. b. brasiliensis. The resulting phylogenies identified two major clades that are congruent with the geographical populations studied. Based on collection sites and in accordance with type-location, one clade was identified as the subspecies T. b. macromelasoma. The second clade grouped T. b. brasiliensis populations. Restriction endonuclease sites were observed in the sequences and used in PCR-RFLP assays, producing distinct fingerprints for T. b. macromelasoma and T. b. brasiliensis populations. The results suggest that these are different species and that gene flow occurs only among T. b. brasiliensis populations, possibly associated with human activity in the area.
1. Introduction
About 28 million people live in areas at risk of Chagas disease, 11–14.5 million of whom are affected worldwide. Trypanosoma cruzi, the pathogen that causes Chagas disease, is found in most South American countries, representing an important cause of heart damage among the economically active population [1]. After a successful chemical control of Triatoma infestans (Klugi, 1834), the other main vectors of Chagas causing agent, Panstrongylus megistus Burmeister, 1835, Rhodnius prolixus Stal, 1859, and Triatoma brasiliensis sensu lato Neiva 1911. T. brasiliensis kept attracting considerable attention from local entomological surveillance.
Triatoma brasiliensis sensu lato (s.l.), found in anthropogenic habitats and considered the main vector in northeast Brazil [2, 3], was recently found to be a species complex that includes T. b. brasiliensis, T. b. macromelasoma Galvão, 1956, T. juazeirensis Costa & Felix, 2007, T. sherlocki Papa, Jurberg, Carcavallo, Cerqueira & Barata, 2002, and T. melanica Costa et al., 2006. These taxa exhibit wide phenotypic and morphological variability, displaying specific ecological requirements and chromatic patterns [4]. In this respect, accurate species identification is necessary for effective vector control.
The systematic of Triatominae species is based on morphological characters of the adult exoskeleton and male phallic structures [5]. However, insects captured during vector monitoring and control or received for identification and notification are often immature. Although their characteristics are similar to those of adult individuals, they are difficult to distinguish. The morphology of Triatominae species is not well described; with studies on the immature forms performed for only 40 species, eggs and nymphs described for only 20 species, a key to identify nymphs to the species level has yet to be developed. Available keys are useful and partially applicable to other stages, but specific identification of all live forms remains unresolved [6].
Members of the T. brasiliensis complex have been distinguished by analyzing isoenzymes [7], mitochondrial DNA sequences [8], and random amplification of polymorphic DNA-RAPD [9]. In the present study we analyzed the barcoding CO1 sequences of nine T. brasiliensis s.l. populations from northeastern Brazil (States of Pernambuco, Paraíba, and Rio Grande do Norte) in order to identify their genetic relationships. We also conducted a PCR-RFLP assay to distinguish between T. b. macromelasoma and T. b. brasiliensis subspecies.
2. Materials and Methods
2.1. Sample Collection
Triatominae were collected by surveillance technicians during active inspections in anthropogenic environments (domestic and peridomestic habitats). Live specimens were collected in nine localities in Northeast Brazil (Table 1, Figure 1) using tweezers, flashlights, and PIRISA. Housed in plastic boxes (7 cm diameter × 8 cm high) lined with folded filter paper, the bugs were transported in coolers to the Culicid and Triatomine Laboratory of the Department of Epidemiology, Faculty of Public Health/USP.
Table 1.
Triatoma brasiliensis collection sites, coordinates, labels of selected sequences, and GenBank access numbers.
| Localities (state) | Coordinates | Label | GenBank |
|---|---|---|---|
| Salgueiro (PE) | 08°04′21.60′′S 39°07′57.39′′W |
15 A/B | JQ088297/JQ088298 |
| Monteiro (PB) | 07°53′29.40′′S 37°07′00.79′′W |
30 A/B | JQ088299/JQ088300 |
| Mãe d'água (PB) | 07°15′10.03′′S 37°25′58.25′′W |
35 A/B | JQ088301/JQ088302 |
| Lagoa Grande (PE) | 08°59′07.10′′S 40°18′20.46′′W |
41 A/B | JQ088303/JQ088304 |
| Caicó (RN) | 06°27′30.69′′S 37°06′09.71′′W |
42 A/B | JQ088305/JQ088306 |
| Serra Talhada (PE) | 07°59′09.46′′S 38°17′37.70′′W |
43 A/B | JQ088307/JQ088308 |
| São José (PB) | 06°52′00.00′′S 38°38′00.00′′W |
53 A/B | JQ088309/JQ088310 |
| Santa Cruz (PB) | 06°31′33.48′′S 38°03′23.52′′W |
54 A/B | JQ088311/JQ088312 |
| São Francisco (PB) | 06°36′53.43′′S 38°05′21.89′′W |
55 A/B | JQ088313/JQ088314 |
Figure 1.
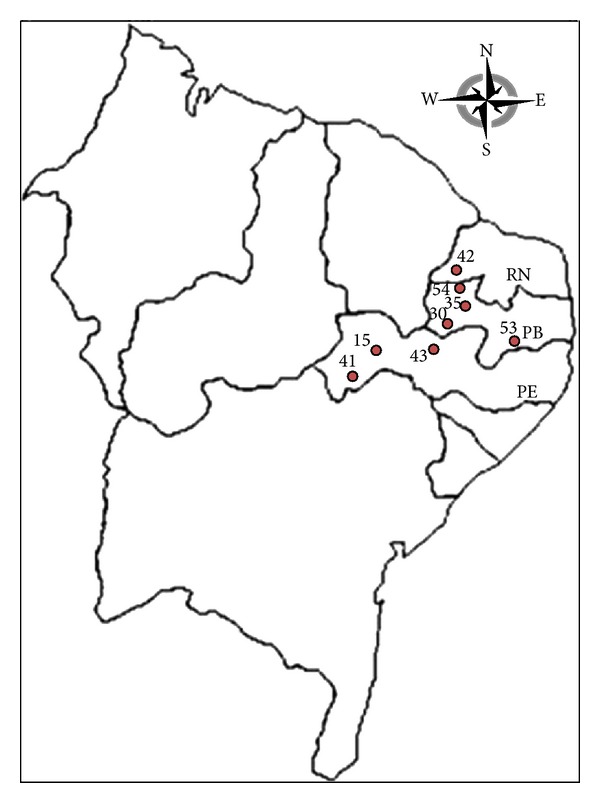
Map of northeast region of Brazil indicating the localities of the Triatoma brasiliensis populations studied. Paraíba State: Monteiro (30), Mãe d'água (35), Santa Cruz (54), and São José (53), São Francisco (55). Pernambuco State: Lagoa Grande (41), Salgueiro (15), and Serra Talhada (43). Rio Grande do Norte State: Caicó (42).
Adults were identified as T. b. brasiliensis according to the key by Lent and Wygodzinsky [5]. Nymphs were assumed to be T. brasiliensis s.l. because, in addition to the difficulty in distinguishing immature Triatominae based on chromatic and morphological characters, comparative material or a key for nymph identification to species level are not available. After morphological identification, DNA was extracted from individuals from the nine localities and sequenced the 520 bp barcode portion of the CO1 gene (Table 1). Specimens collected in Pernambuco State (even nymphs) were assumed to be T. b. macromelasoma, according to Costa et al. [10] and Costa et al. [11].
2.2. DNA Extraction and CO1 Amplification
Genomic DNA was extracted from the legs of 10 individual samples of each population using the Qiagen DNeasy Blood and Tissue Kit (Qiagen, Crawley, United Kingdom) following the manufacturer's protocol. The CO1 barcode region was amplified from whole genomic DNA using primers LCOI 1490 (5′-GGT CAA CAA ATC ATA AAG ATA TTG G-3′) and HCOI 2198 (5′-TAA ACT TCA GGG TGA CCA AAA AAT-3′) [12].
PCR amplification was carried out in a final volume of 50 μL containing PCR buffer, 0.2 mM of each dNTP, 2.5 mM MgCl2, and 1.25 units of Taq polymerase. Initial PCR denaturation was at 94°C for 5 min, followed by 40 cycles of denaturation (1 min) at 94°C, annealing (2 min) at 50°C, and extension (2 min) at 72°C. A final extension step at 72°C was performed for 10 min. The amplified DNA was loaded onto 1.5% agarose gel and stained with ethidium bromide. Amplicons were sequenced in both forward and reverse directions using ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction Kits (Perkin Elmer, Foster City, CA) on an ABI PRISM 3100 Genetic Analyzer/HITACHI.
2.3. Analysis of DNA Sequence
Sequences were aligned using Clustal W [13]. One or two representative haplotypes for each population was chosen because of the low variation within populations. Phylogenetic reconstructions were performed by Neighbor Joining and Maximum Likelihood methods (both using the Kimura-3-parameter distance model K81) in MEGA 5.0 [14], and a divergence matrix was constructed under Kimura two parameters (K2P) (Table 2). Maximum Parsimony was carried out using the branch-and-bound search option. Phylogenetic analyses included 1000 bootstrap replicates and a Triatoma sordida CO1 sequence (Genbank acc. no. AF021213) as outgroup. Triatoma brasiliensis s.l. CO1 sequences were also analyzed using NEBcutter version 2.0 [15] to select appropriate endonuclease enzymes.
Table 2.
Matrix of divergence of CO1 gene fragment of T. brasiliensis specimens using K2P model.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) 15A Salgueiro PE | |||||||||||||||||||
| (2) 15B Salgueiro PE | 0.000 | ||||||||||||||||||
| (3) 30A Monteiro PB | 0.039 | 0.039 | |||||||||||||||||
| (4) 30B Monteiro PB | 0.039 | 0.039 | 0.000 | ||||||||||||||||
| (5) 35A Me d'gua PB | 0.037 | 0.037 | 0.001 | 0.001 | |||||||||||||||
| (6) 35B Ma d'gua PB | 0.037 | 0.037 | 0.001 | 0.001 | 0.000 | ||||||||||||||
| (7) 41A Lagoa Grande PE | 0.031 | 0.031 | 0.013 | 0.013 | 0.011 | 0.011 | |||||||||||||
| (8) 41B Lagoa Grande PE | 0.031 | 0.031 | 0.013 | 0.013 | 0.011 | 0.011 | 0.000 | ||||||||||||
| (9) 42A Caic RN | 0.037 | 0.037 | 0.001 | 0.001 | 0.000 | 0.000 | 0.011 | 0.011 | |||||||||||
| (10) 42B Caic RN | 0.037 | 0.037 | 0.001 | 0.001 | 0.000 | 0.000 | 0.011 | 0.011 | 0.000 | ||||||||||
| (11) 43A Serra Talhada PE | 0.039 | 0.039 | 0.003 | 0.003 | 0.001 | 0.001 | 0.013 | 0.013 | 0.001 | 0.001 | |||||||||
| (12) 43B Serra Talhada PE | 0.039 | 0.039 | 0.003 | 0.003 | 0.001 | 0.001 | 0.013 | 0.013 | 0.001 | 0.001 | 0.000 | ||||||||
| (13) 53A São Jos PB | 0.037 | 0.037 | 0.001 | 0.001 | 0.000 | 0.000 | 0.011 | 0.011 | 0.000 | 0.000 | 0.001 | 0.001 | |||||||
| (14) 53B São Jos PB | 0.037 | 0.037 | 0.001 | 0.001 | 0.000 | 0.000 | 0.011 | 0.011 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | ||||||
| (15) 54A Santa Cruz PB | 0.037 | 0.037 | 0.001 | 0.001 | 0.000 | 0.000 | 0.011 | 0.011 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | |||||
| (16) 54B Santa Cruz PB | 0.037 | 0.037 | 0.001 | 0.001 | 0.000 | 0.000 | 0.011 | 0.011 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | ||||
| (17) 55A São Francisco PB | 0.037 | 0.037 | 0.001 | 0.001 | 0.000 | 0.000 | 0.011 | 0.011 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | |||
| (18) 55B São Francisco PB | 0.039 | 0.039 | 0.003 | 0.003 | 0.001 | 0.001 | 0.013 | 0.013 | 0.001 | 0.001 | 0.003 | 0.003 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | ||
| (19) AF021213.1 Triatoma_sordida | 0.094 | 0.094 | 0.099 | 0.099 | 0.097 | 0.097 | 0.096 | 0.096 | 0.097 | 0.097 | 0.099 | 0.099 | 0.097 | 0.097 | 0.097 | 0.097 | 0.097 | 0.095 | |
| (20) AF021186.1 Triatoma_brasiliensis | 0.026 | 0.026 | 0.014 | 0.014 | 0.013 | 0.013 | 0.007 | 0.007 | 0.013 | 0.013 | 0.014 | 0.014 | 0.013 | 0.013 | 0.013 | 0.013 | 0.013 | 0.014 | 0.105 |
2.4. Enzyme Restriction Analysis
Individual CO1 sequences were PCR-amplified using the above parameters and digested in a 10 μL reaction with StyI (Promega) and HincII (New England Biolabs, Ipswich, MA) enzymes. The reaction contained 1 μL of 10x buffer, 4 μL deionized water, 4 μL of amplification product, and 1 unit of restriction enzyme. The digestion mixture was incubated at 37°C for 2 h and then resolved on 2.0% agarose gel.
3. Results
Phylogenetic trees derived from the Neighbor Joining, Maximum Likelihood, and Parsimony methods showed similar topologies (data shown for ML tree in Figure 2). Since most of CO1 sequences were identical into and among the populations, the ML tree was constructed using only two samples of each one. This ML tree and the Nucleotide Distance Matrix (Table 2) indicated sequence divergence of up to 4% between the two main clades. The basal clade, with about 4% divergence from the other populations, consisted of the Salgueiro population (15A/B). This population was considered to be T. b. macromelasoma due to its high sequence divergence compared to the other populations and because it was collected in its type locality [16]. The second clade, with pairwise distances up to 1%, showed that Pernambuco populations are more basal, although those from Serra Talhada clustered with Paraíba populations likely because of the city's proximity to the Pernambuco-Paraíba border. Paraíba populations formed a large cluster that also included the Rio Grande do Norte population.
Figure 2.
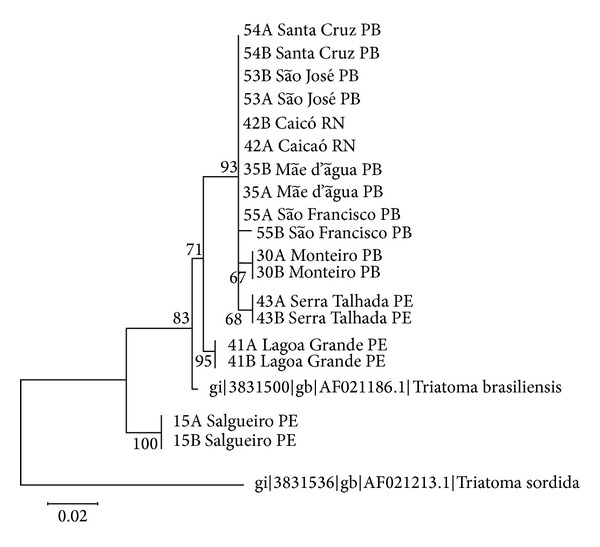
Maximum Likelihood phylogeny generated from CO1 sequences using the Kimura-2-parameter model with 1000 bootstrap replicates. Sequences were rooted with T. sordida (GenBank acc. no.: AF021213).
Based on sequence analysis, a PCR-RFLP assay was performed to differentiate between the subspecies T. b. macromelasoma and T. b. brasiliensis. PCR fragment digestion using the StyI enzyme produced two restriction fragments (342 bp and 192 bp) in CO1 sequences from Salgueiro samples and only one fragment in samples of the other eight populations. Conversely, the HincII enzyme yielded two fragments (297 bp and 240 bp) in all population samples (n = 10 samples of each population) except that from Salgueiro (Figure 3). StyI and HincII enzymes therefore produced distinct fingerprints for T. b. macromelasoma and T. b. brasiliensis, suggesting that they are different subspecies. The molecular protocols described above are a useful tool in the study of populations and cryptic species, contributing to the identification of insect vectors.
Figure 3.
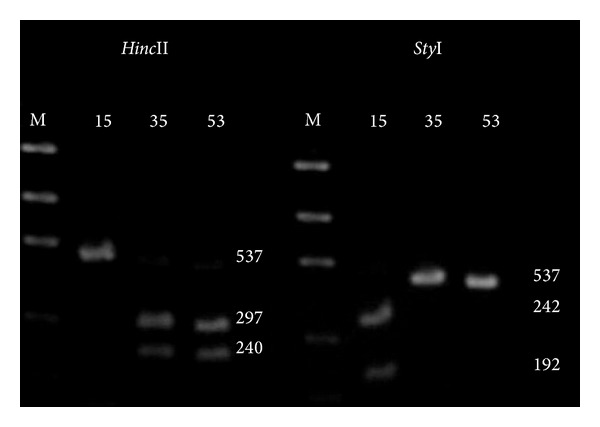
PCR-RFLP fingerprint of T. brasiliensis from three localities produced with StyI and HincII enzymes on 2.0% agarose gel. M: DNA ladder 100 bp (New England Biolabs), 15: Salgueiro, 35: Mãe d'água, and 53: São José.
4. Discussion
The identification of adult Triatominae based on morphological and chromatic pattern is considered relatively easy for most species; however, this is commonly misguided owing to the wide phenotypic variability within this subfamily. For instance, Triatoma maculata Erichson, 1848 and Triatoma pseudomaculata Corrêa & Espínola, 1964 which were first treated as members of a same species complex due to morphological similarities [5] thereafter proved to be genetically distant [17–19]. Panstrongylus herreri Wygodzinsky, 1948 and Panstrongylus lignarius Walker, 1873 in turn, were considered to be separate species until Marcilla et al. [20] and Crossa et al. [21] demonstrated that they are the same species, cytogenetically identical with regard to the second internal transcribed spacer. Another difficulty in identifying genera and species of Triatominae is their extensive chromatic variability. The color of some species such as Rhodnius sp. (light brown tones) and Rhodnius nasutus Stål, 1859 (pinkish tones) seems to be associated with the color of the palm trees they colonize [22], but others such as Triatoma rubrovaria Blanchard, 1843, exhibit well-known 4 chromatic morphotypes [23]. Other studies report the occurrence of natural homoploid hybrids between T. infestans and Triatoma platensis Neiva, 1913, T. infestans and Triatoma rubrovaria, and sympatric species of Phyllosoma complex and species of the T. brasiliensis complex. In addition, several hybrid species have been obtained experimentally [24]. This interspecific crossing can be decisive in originating and diversifying wild species, resulting in important epidemiological consequences due to differential competence and the capacity of hybrid vectors [3, 10, 11]. Therefore, the characterization (or identification) of Triatominae specimens based only on morphological and chromatic patterns, the most common identification method, is more complex than previously believed.
Studies on immature stages are crucial for group systematics. However, literature reports on immature forms of certain groups are scarce, difficult to use, or nonexistent. Many species undergo changes in color, structure, and morphology during their development, hindering their identification [25, 26]. Triatominae nymphs at this development stage are difficult to identify. To that end, molecular analyses are successfully used to characterize morphotypes of species complexes such as T. brasiliensis sp., which exhibits wide chromatic and morphological variation [7, 8].
Marked differences in color pattern and ecological features among species from the Triatoma brasiliensis complex were detected by microsatellites, mitochondrial 12S, and cytochrome b genes, reinforcing species diagnosis [7, 16]. However, individuals from subspecies T. b. brasiliensis and T. b. macromelasoma might be clustered within the same CO1 clade, since earlier studies have shown that some cytochrome b haplotypes of T. b. macromelasoma are similar to those of T. b. brasiliensis [16]. Moreover, these subspecies produce fertile hybrids when crossed in laboratory [3].
The basal clade of the Maximum Likelihood tree (Figure 1) was identified as subspecies T. b. macromelasoma because it is highly divergent (6%, as shown in the Nucleotide Distance Matrix, Table 2) from the other populations and was collected in its type locality [16]. On the other hand, all populations in the second clade were identified as T. b. brasiliensis. Their genetic similarity may be related to geographic proximity and similar habitat conditions. However, interpopulation divergence values (<1%) suggest that T. b. brasiliensis is still diversifying and/or exhibiting ongoing gene flow, probably due to human-assisted dispersal. Based on wing morphometry, Costa et al. [11] recently formulated a hypothesis that T. b. macromelasoma is the result of homoploidal hybridization between T. b. brasiliensis and T. juazeirensis in the state of Pernambuco, and that this is a form of speciation in sympatric populations.
In northeastern Brazil, the epidemiological importance of Triatominae bugs is mainly defined by their high rate of natural T. cruzi infection and ability to adapt to multiple ecotopes. Control measures therefore require a precise identification of which species of the T. brasiliensis complex is being targeted. Moreover, it is important to understand the ecoepidemiology of Triatominae since these vectors are found in large numbers in their natural habitat [27]. In this respect, the PCR-RFLP protocol described here is suggested as rapid, relatively simple, and economical assay to distinguish Triatoma b. macromelasoma from Triatoma b. brasiliensis subspecies. Even at small geographic scales, domestic populations are genetically structured by ecological parameters, thereby exhibiting small differences from the wild counterparts from which they are derived [8]. The present study highlights the effectiveness of the CO1 gene in identifying subspecies of the T. brasiliensis complex and its contribution to classic taxonomy.
Disclosure
No competing financial interests exist in the paper.
Acknowledgments
The authors thank Dr. Maria Helena Matté (FSP/USP) for providing the restriction enzymes. Financial support was provided by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). Daniel Pagotto Vendrami is a Master's student sponsored by FAPESP (2010/02960-3).
References
- 1.Moncayo Á, Silveira AC. Current epidemiological trends for Chagas disease in Latin America and future challenges in epidemiology, surveillance and health policy. Memorias do Instituto Oswaldo Cruz. 2009;104(1):17–30. doi: 10.1590/s0074-02762009000900005. [DOI] [PubMed] [Google Scholar]
- 2.Silveira AC, Vinhaes MC. Elimination of vector-borne transmission of chagas disease. Memorias do Instituto Oswaldo Cruz. 1999;94(1):405–411. doi: 10.1590/S0074-02761999000700080. [DOI] [PubMed] [Google Scholar]
- 3.Costa J, Almeida CE, Dujardin JP, Beard CB. Crossing experiments detect genetic incompatibility among populations of Triatoma brasiliensis Neiva, 1911 (Heteroptera, Reduviidae, Triatominae) Memorias do Instituto Oswaldo Cruz. 2003;98(5):637–639. doi: 10.1590/s0074-02762003000500009. [DOI] [PubMed] [Google Scholar]
- 4.Mendonça VJ, Da Silva MTA, De Araújo RF, et al. Phylogeny of Triatoma sherlocki (Hemiptera: Reduviidae:Triatominae) inferred from two mitochondrial genes suggests its location within the Triatoma brasiliensis complex. American Journal of Tropical Medicine and Hygiene. 2009;81(5):858–864. doi: 10.4269/ajtmh.2009.08-0664. [DOI] [PubMed] [Google Scholar]
- 5.Lent H, Wygodzinsky P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas' disease. Bulletin of the American Museum of Natural History. 1979;16:123–520. [Google Scholar]
- 6.Galvão C, McAloon FM, Rocha DS, Schaefer CW, Patterson J, Jurberg J. Description of eggs and nymphs of Linshcosteus karupus (Hemiptera: Reduviidae: Triatominae) Annals of the Entomological Society of America. 2005;98(6):861–872. [Google Scholar]
- 7.Costa J, Freitas-Sibajev MGR, Marchon-Silva V, Pires MQ, Pacheco RS. Isoenzymes Detect Variation in Populations of Triatoma brasiliensis (Hemiptera: Reduviidae: Triatominae) Memorias do Instituto Oswaldo Cruz. 1997;92(4):459–464. doi: 10.1590/s0074-02761997000400002. [DOI] [PubMed] [Google Scholar]
- 8.Almeida CE, Pacheco RS, Haag K, Dupas S, Dotson EM, Costa J. Inferring from the Cyt B gene the Triatoma brasiliensis Neiva, 1911 (Hemiptera: Reduviidae: Triatominae) genetic structure and domiciliary infestation in the State of Paraíba, Brazil. American Journal of Tropical Medicine and Hygiene. 2008;78(5):791–802. [PubMed] [Google Scholar]
- 9.Borges EC, Romanha AJ, Diotaiuti L. Use of random amplified polymorphic DNA (RAPD) in the populational study of Triatoma brasiliensis Neiva, 1911. Cadernos de Saúde Pública. 2000;16:97–100. [PubMed] [Google Scholar]
- 10.Costa J, Almeida CE, Dotson EM, et al. The epidemiologic importance of Triatoma brasiliensis as a chagas disease vector in Brazil: a revision of domiciliary captures during 1993–1999. Memorias do Instituto Oswaldo Cruz. 2003;98(4):443–449. doi: 10.1590/s0074-02762003000400002. [DOI] [PubMed] [Google Scholar]
- 11.Costa J, Peterson AT, Dujardin JP. Morphological evidence suggests homoploid hybridization as a possible mode of speciation in the Triatominae (Hemiptera, Heteroptera, Reduviidae) Infection, Genetics and Evolution. 2009;9(2):263–270. doi: 10.1016/j.meegid.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular marine biology and biotechnology. 1994;3(5):294–299. [PubMed] [Google Scholar]
- 13.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincze T, Posfai J, Roberts RJ. NEBcutter: a program to cleave DNA with restriction enzymes. Nucleic Acids Research. 2003;31(13):3688–3691. doi: 10.1093/nar/gkg526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monteiro FA, Donnelly MJ, Beard CB, Costa J. Nested clade and phylogeographic analyses of the Chagas disease vector Triatoma brasiliensis in Northeast Brazil. Molecular Phylogenetics and Evolution. 2004;32(1):46–56. doi: 10.1016/j.ympev.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Belisário CJ, Pessoa GCD, Diotaiuti L. Biological aspects of crosses between Triatoma maculata (Erichson, 1848) and Triatoma pseudomaculata Corrêa & Espínola, 1964 (Hemiptera: Reduviidae) Memorias do Instituto Oswaldo Cruz. 2007;102(4):517–521. doi: 10.1590/s0074-02762007005000029. [DOI] [PubMed] [Google Scholar]
- 18.dos Santos SM, Lopes CM, Dujardin JP, et al. Evolutionary relationships based on genetic and phenetic characters between Triatoma maculata, Triatoma pseudomaculata and morphologically related species (Reduviidae: Triatominae) Infection, Genetics and Evolution. 2007;7(4):469–475. doi: 10.1016/j.meegid.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho-Costa FA, Dos Santos SM, Pires MQ, Lopes CM, Noireau F, Pacheco RS. Sylvatic and peridomestic populations of Triatoma pseudomaculata are not significantly structured by habitat, as revealed by two genetic markers. Journal of Vector Ecology. 2010;35(2):295–300. doi: 10.1111/j.1948-7134.2010.00085.x. [DOI] [PubMed] [Google Scholar]
- 20.Marcilla A, Bargues MD, Abad-Franch F, et al. Nuclear rDNA ITS-2 sequences reveal polyphyly of Panstrongylus species (Hemiptera: Reduviidae: Triatominae), vectors of Trypanosoma cruzi . Infection, Genetics and Evolution. 2002;1(3):225–235. doi: 10.1016/s1567-1348(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 21.Crossa RP, Hernández M, Caraccio MN, et al. Chromosomal evolution trends of the genus Panstrongylus (Hemiptera, Reduviidae), vectors of Chagas disease. Infection, Genetics and Evolution. 2002;2(1):47–56. doi: 10.1016/s1567-1348(02)00063-1. [DOI] [PubMed] [Google Scholar]
- 22.Dias FBS, Paula ASD, Belisário CJ, et al. Influence of the palm tree species on the variability of Rhodnius nasutus Stål, 1859 (Hemiptera, Reduviidae, Triatominae) Infection, Genetics and Evolution. 2011;11(5):869–877. doi: 10.1016/j.meegid.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Almeida CE, Pacheco RS, Noireau F, Costa J. Triatoma rubrovaria (Blanchard, 1843) (Hemiptera: Reduviidae) I: isoenzymatic and chromatic patterns of five populations from the State of Rio Grande do Sul, Brazil. Memorias do Instituto Oswaldo Cruz. 2002;97(6):829–834. doi: 10.1590/s0074-02762002000600013. [DOI] [PubMed] [Google Scholar]
- 24.Correia N, Almeida CE, Lima-Neiva V, et al. Cross-mating experiments detect reproductive compatibility between Triatoma sherlocki and other members of the Triatoma brasiliensis species complex. Acta Tropica. 2013;128:162–167. doi: 10.1016/j.actatropica.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 25.van Emden FT. The taxonomic significance of the characters of immature insects. Annual Review of Entomology. 1957;2:91–106. [Google Scholar]
- 26.Costa J, Argolo AM, Felix M. Taken of Triatoma melanica Neiva & Lent, 1941, new status (Hemiptera: Reduviidae: Triatominae) Zootaxa. 2006;1385:47–52. [Google Scholar]
- 27.Ramsey JM, Schofield CJ. Control of Chagas disease vectors. Salud Publica de Mexico. 2003;45(2):123–128. doi: 10.1590/s0036-36342003000200010. [DOI] [PubMed] [Google Scholar]


