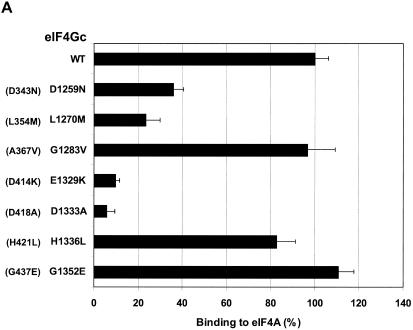
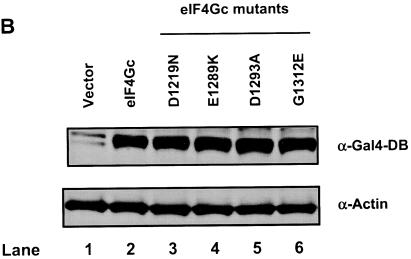
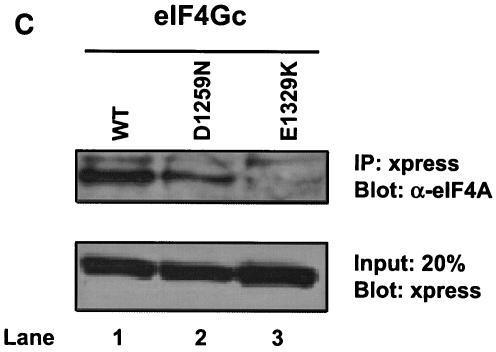
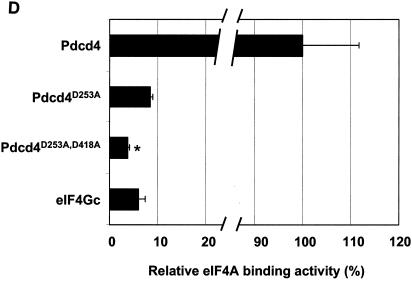
FIG. 3.
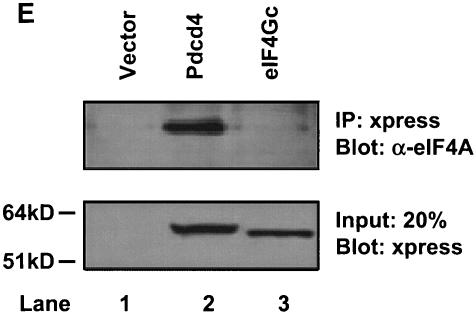
eIF4Gc has an MA-3 domain that is functional for eIF4A binding. (A) Plasmid pCMV-BD-eIF4Gc (50 ng) (wild type [WT] or site-specific mutant) was transiently transfected with pCMV-AD-eIF4A (400 ng) and Gal4-luciferase reporter DNA (25 ng) into JB6 RT101 cells. (B) Similar expression of wild-type and mutant Gal4 DNA-binding domain-eIF4Gc. RT101 cell lysates (10 μg) from a transient transfection with wild type (lane 2) and site-specific mutants (lanes 3 to 6) pCMV-BD-eIF4Gc expression plasmids were separated on 10% Bis-Tris NuPage gels, transferred to nitrocellulose, and subjected to immunoblotting with Gal4 DNA-binding domain antibody, with visualization by chemiluminescent detection. (C) Coimmunoprecipitation of eIF4A with xpress-tagged eIF4Gc. RT101 cell lysates from transient transfection with xpress-tagged eIF4Gc (lane 1), xpress-tagged eIF4GcD1259N (lane 2), or xpress-tagged eIF4GcE1329K (lane 3) expression plasmids were immunoprecipitated with mouse monoclonal xpress antibody. The bound proteins were resolved on a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with eIF4A or xpress antibody. (D) Plasmid pCMV-BD-Pdcd4 (50 ng) (wild type [WT] or point mutation mutants) or pCMV-BD-eIF4Gc (50 ng) was transiently transfected with pCMV-AD-eIF4A (50 ng) and Gal4-luciferase reporter DNA (25 ng) into JB6 RT101 cells. After 48 h, cells were lysed and the luciferase activity was measured. The luciferase activity with wild-type Pdcd4 was designated as 100%. These experiments were repeated three times with five independent transfections, and representative data are shown. The results are expressed as the means plus the standard deviations. An asterisk indicates a significant difference compared to the transfection with pCMV-BD-eIF4Gc, as determined by Student t test (P < 0.005). (E) Relative eIF4A-binding activity of eIF4Gc. RT101 cell lysates from a transient transfection with empty vector (lane 1) xpress-tagged Pdcd4 expression plasmid (lane 2) or xpress-tagged eIF4Gc expression plasmid (lane 3) were immunoprecipitated with mouse monoclonal xpress antibody. The bound proteins were resolved on a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with eIF4A or xpress antibody.