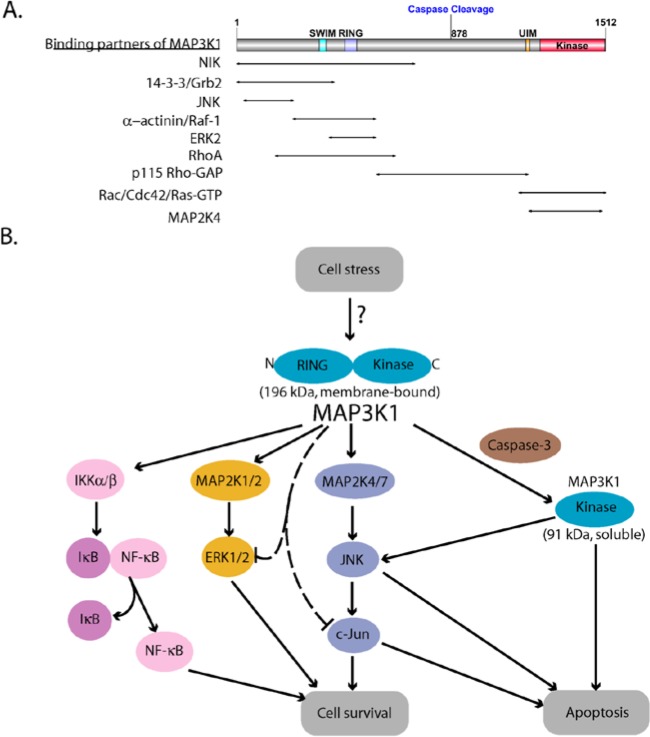
Figure 1.
MAP3K1 domain organization and dual roles in cell survival and apoptosis. (A) Schematic structure of MAP3K1. It contains a SWIM and a RING zinc finger domain near the N-terminus and a serine/threonine kinase domain at the C-terminus. Caspase-3 cleavage occurs at the aspartate 874 of the mouse MAP3K1, which is equivalent to residue 878 of the human homolog. The protein domain structure was created using DOG 1.0 program.17 MAP3K1 also harbors binding sites for multiple upstream and downstream proteins indicated by the arrows. (B) MAP3K1 has a switch-like function that determines cell fate. Activation of MAP2K4/7-JNK-c-Jun, MAP2K1/2-ERK1/2, and NF-κB mediated by full length MAP3K1 promotes cell survival while caspase cleavage, which generates the soluble active kinase domain, induces apoptosis. MAP3K1 also ubiquitylates c-Jun and ERK1/2, leading to their degradation.