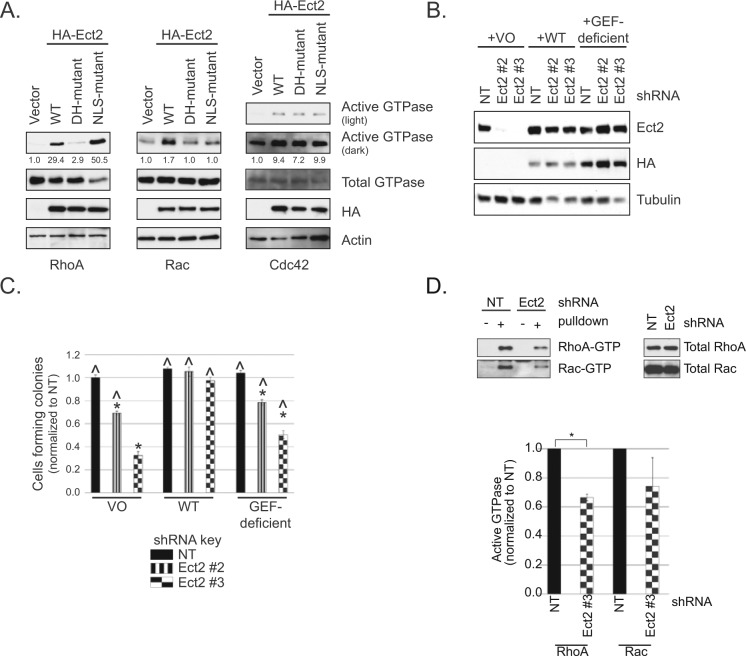
Figure 4.
RhoGEF activity is required for Ect2-mediated transformed growth. (A) A catalytic DH-domain mutant (E428A/N608A) of Ect2 is impaired in RhoGEF activity on RhoA and Rac1 but not Cdc42. Ectopic expression of HA-tagged wild type (WT) but not mutant Ect2 increased active RhoA-GTP or Rac1-GTP in whole cell lysates, as shown by pulldown assays using the Rho binding domain (RBD) of effectors that selectively bind the active, GTP-bound form of each GTPase (293T cells; anti-HA, 1:1,000, clone 3F10, Roche, Indianapolis, IN; anti-RhoA, 1:1,000, clone 67B9, Cell Signaling, Danvers, MA; anti-Rac1, 1:1,000, clone 23A8, Millipore; anti-Cdc42, 1:500, clone B-8, Santa Cruz, Dallas, TX). In the same assay, overexpression of Ect2 with mutated nuclear localization signals (NLS) caused a dramatic increase in RhoA activity but not Rac activity. Ect2 activation of Cdc42 was unaffected by either DH or NLS mutations. Percent activation is shown based on densitometry performed on each active GTPase blot and normalized to total GTPase (representative of n ≥ 3). (B) Re-expression of WT or the GEF-deficient DH-mutant Ect2 in knockdown cells restores Ect2 levels to similar degrees. The shRNA-resistant mutants indicated above were stably expressed in the Ect2 knockdown OVCAR8 cells and immunoblotted with anti-HA antibody for ectopic Ect2 and with anti-Ect2 antibody for endogenous + ectopic Ect2. Tubulin served as a loading control. (C) Re-expression of WT but not GEF-deficient Ect2 rescues anchorage-independent growth. Error bars represent SEM. The baseline of colony counts was considered to be those seen in cells where Ect2 was not knocked down (NT) and expression was “rescued” with vector-only (VO). Colony counts statistically significant from this baseline are marked with an asterisk * (P < 0.05, with Bonferroni correction). The ability of ectopic Ect2 to rescue Ect2 knockdown was evaluated by comparison to cells expressing Ect2 shRNA#3 and “rescued” with vector-only (^, P < 0.05, with Bonferroni correction; n = 5). (D) Ect2 knockdown results in decreased levels of active RhoA and Rac in whole cell lysate. Standard pulldown assays were performed using the Rho binding domain (RBD) of effectors that selectively bind the active, GTP-bound form of RhoA (Rhotekin-RBD) and Rac (PAK-RBD). Upper panel = representative pulldown; lower panel = average of all assays (n ≥ 4). Error bars represent SEM and * represents P < 0.05, based on a paired t-test in which values were log-transformed for normality.