Abstract
Self-disturbances (SDs) are increasingly identified in schizophrenia and are theorized to confer vulnerability to psychosis. Neuroimaging research has shed some light on the neural correlates of SDs in schizophrenia. But, the onset and trajectory of the neural alterations underlying SDs in schizophrenia remain incompletely understood. We hypothesize that the aberrant structure and function of brain areas (e.g., prefrontal, lateral temporal, and parietal cortical structures) comprising the “neural circuitry of self” may represent an early, premorbid (i.e., pre-prodromal) indicator of schizophrenia risk. Consistent with neurodevelopmental models, we argue that “early” (i.e., perinatal) dysmaturational processes (e.g., abnormal cortical neural cell migration and mini-columnar formation) affecting key prefrontal (e.g., medial prefrontal cortex), lateral temporal cortical (e.g., superior temporal sulcus), parietal (e.g., inferior parietal lobule) structures involved in self-processing may lead to subtle disruptions of “self” during childhood in persons at risk for schizophrenia. During adolescence, progressive neurodevelopmental alterations (e.g., aberrant synaptic pruning) affecting the neural circuitry of self may contribute to worsening of SDs. This could result in the emergence of prodromal symptoms and, eventually, full-blown psychosis. To highlight why adolescence may be a period of heightened risk for SDs, we first summarize the literature regarding the neural correlates of self in typically developing children. Next, we present evidence from neuroimaging studies in genetic high-risk youth suggesting that fronto-temporal-parietal structures mediating self-reflection may be abnormal in the premorbid period. Our goal is that the ideas presented here might provide future directions for research into the neurobiology of SDs during the pre-psychosis development of youth at risk for schizophrenia.
Keywords: self-disturbances, schizophrenia, premorbid, genetic high-risk, neurodevelopment
1. Introduction
1.1 Self-disturbances in schizophrenia and the psychosis prodrome
Self-disturbances (SDs) are increasingly recognized in schizophrenia (Vogeley, 2007) and are thought to be a core feature of the psychopathology of the illness (Sass and Parnas, 2003; Vinogradov et al., 2008). Consistent with this hypothesis, phenomenological research indicates that anomalies of “self-experience” (e.g., alterations of the sense of being the subject of one’s own experiences) show greater specificity for schizophrenia than for other psychotic-spectrum disorders (e.g., bipolar disorder) (Parnas et al., 2005; Parnas et al., 2003). Further, neuropsychological studies in people with schizophrenia have increasingly linked impairments across a range of conceptually-related mental processes that implicitly, or explicitly involve self-reflection (e.g., metacognition, theory of mind [ToM], and reality monitoring) with: 1) key psychotic symptoms (i.e., delusions (Frith and Corcoran, 1996; Langdon et al., 1997), hallucinations (Johns et al., 2006; Keefe et al., 2002)); 2) poor insight into illness (Bora et al., 2007; Koren et al., 2004); and 3) greater social dysfunction (Fett et al., 2011; Lysaker et al., 2005). Moreover, because neuropsychological deficits of self-monitoring may result in confusion regarding the discrimination between self and other, it is theorized that they may also underlie first-rank symptoms ([FRS (Schneider, 1959)]; e.g, thought insertion, delusions of influence, voices commenting), a possibly pathognomonic feature of schizophrenia (Stephan et al., 2009).
Recent evidence additionally suggests that disruptions of self-experience (Nelson et al., 2012) and/or self-related processing (Bora and Pantelis, 2013; Kim et al., 2011) may confer an increased vulnerability to the development of psychosis. For example, several studies have shown that SDs (e.g., anomalies of self-experience (Koren et al., 2012; Nelson et al., 2012)), as well impairments of ToM (Bora and Pantelis, 2013) are linked with the “psychosis prodrome” and also may be predictive of a greater risk of transitioning to full-blown psychosis, although it is not entirely clear if this is specific to the psychosis of schizophrenia (Kim et al., 2011; Nelson et al., 2012). Thus, determining the neurobiological basis of SDs could contribute to the development of a biological marker for psychosis and particularly schizophrenia risk that might be useful for the enhancement of early intervention/prevention strategies for at-risk individuals.
1.2 The neural circuitry of self-reflective processing
The aspect of “self” that is disrupted in persons with schizophrenia remains the focus of ongoing academic debate (Cermolacce et al., 2007). Phenomenological theorists suggest that schizophrenia may have its basis in disturbances of the “minimal self” – i.e., a “pre-reflexive,” core sense of being the subject of one’s experiences (Sass and Parnas, 2003). Alternatively, cognitive neuroscience research has tended to focus attention on disturbances of “higher-order” mental capacities involved in self-awareness (e.g., self-representation and self-monitoring) in schizophrenia (Kircher and Leube, 2003; Newen and Vogeley, 2003). Nevertheless, it is theorized that because alterations occurring at various “levels” of self-structure are likely to be inter-dependent (Kircher and Leube, 2003; Newen and Vogeley, 2003; Parnas, 2003), they may plausibly arise from common underlying neurobiological mechanisms (Nelson et al., 2009). Evidence from functional magnetic resonance imaging (fMRI) studies in healthy subjects has provided consistent evidence that “self” processing involves the activation of cortical structures comprising a fronto-temporal-parietal network (e.g., ventral and dorsal medial prefrontal cortex (MPFC), anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), superior temporal sulcus (STS), and inferior parietal cortex (Frith and Frith, 2003; Gallagher et al., 2000; Jenkins and Mitchell, 2011; Kelley et al., 2002; Saxe et al., 2004; Torrey, 2007). For example, recruitment of midline structures (MPFC, PCC) has been linked with introspection (Mitchell, 2009) and the retrieval of autobiographical memory (Summerfield et al., 2009). Lateral temporal cortical activation (particularly posterior STS) has been consistently associated with tasks involving reflection on the intentions and mental states of others (Saxe and Kanwisher, 2003). Additionally, inferior parietal cortex, which is engaged during somatosensory processing and the integration of sensory input, has been implicated in self-perception (e.g., bodily self-awareness) and the differentiation between self and other (Herwig et al., 2012; Torrey, 2007).
Further, these fronto-temporal-parietal cortical structures are increasingly thought to be part of a more widely-distributed neural network involved in self-referential and self-other discriminative processing, including other parts of the prefrontal cortex ([PFC]; e.g., inferior frontal gyrus (D’Argembeau et al., 2013; Morin and Hamper, 2012), dorsolateral PFC (Herwig et al., 2012; Pauly et al., 2012; Schmitz et al., 2004)), the temporal poles (Blackwood et al., 2004; Pauly et al., 2012), and insula (van der Meer et al., 2010) – what we will term for short-hand the “neural circuitry of self.” Typically, the engagement of these brain areas during self-reflection is indicated by their increased activation during the retrieval of self-specific information, in contrast to their levels of activation during tasks that involve focusing on physical, or semantic aspects of stimuli (Amodio and Frith, 2006). Additionally, key components of this neural circuitry (most notably MPFC and PCC, but also parts of the lateral temporal and parietal cortices) show increased activity (Gusnard et al., 2001a; Gusnard et al., 2001b; Raichle et al., 2001) and functional coupling (Greicius et al., 2003; Greicius and Menon, 2004) “at rest” (i.e., in the absence external stimuli) and are considered “nodes” of the brain’s “default mode network” (DMN) (Buckner et al., 2008). Fluctuations in the blood oxygen level-dependent (BOLD) signal during rest are believed to reflect the intrinsic functional organization of the brain (Whitfield-Gabrieli and Ford, 2012), providing additional reason to believe that these structures may function to support “internal mentation,” or introspective processing (Buckner et al., 2008).
1.3 The neural correlates of impaired self-processing in schizophrenia
Neuroimaging research has begun to shed some light on the neural correlates of SDs in schizophrenia, and related schizophrenia disorders. Resting-state fMRI studies in schizophrenia, for example, have shown aberrant functional connectivity both within (intra-PFC) and between (MPFC-PCC) the same midline cortical structures that are consistently activated during self-reflective processing in healthy subjects (Karbasforoushan and Woodward, 2012). Further, dysfunction of the neural circuitry of self, including abnormal activation of midline (i.e., MPFC and PCC (Blackwood et al., 2004; Brunet et al., 2003; Holt et al., 2011; Russell et al., 2000)), lateral temporal (i.e., STS (Brune et al., 2008; Murphy et al., 2010; Wang et al., 2011)), and parietal (i.e., inferior parietal lobule (Bedford et al., 2012; Jardri et al., 2011) cortical structures, is increasingly reported during tasks involving explicit, or implicit self-reflective processing in schizophrenia. Despite the growing theoretical and empirical evidence linking SDs in schizophrenia to underlying alterations of the functioning of the neural circuitry of self, the timing and trajectory of these neural abnormalities with respect to the onset of psychosis, remains incompletely understood.
As we show below, however, there is some evidence suggesting that alterations of the brain structures mediating self-reflective processing could reflect premorbid risk markers whose roots stem from disturbances of early neurodevelopment, and are not simply associated with psychosis per se. We hypothesize that SDs may represent an early, premorbid (i.e., pre-prodromal) indicator of schizophrenia risk that results from abnormalities of the structure and function of the neural circuitry of self occurring during childhood in persons who later develop schizophrenia. Based on neurodevelopmental models of schizophrenia (Keshavan et al., 1994; Keshavan and Hogarty, 1999), we propose that the developmental trajectory of SDs may evolve as the result of a combination of “early” (i.e., perinatal) and “late” (i.e., adolescent/early adult) brain dysmaturational processes affecting the brain structures involved in self-processing.
To provide a context for our discussion of the putative abnormalities of this neural system in youth at risk for schizophrenia, and to highlight why the transition to adolescence may be a time of heightened risk for SDs, we first begin with a summary of the literature regarding the neural correlates of self in typically developing children. Next, we present evidence from neuroimaging studies in genetic high-risk (GHR) individuals (with a focus on youth, age 30 or less) suggesting 1) that brain structures mediating self-reflection and/or self-other discrimination may be abnormal in the premorbid period; and 2) that the abnormalities of brain areas linked with the neural circuitry of self may have a progressive neurodevelopmental trajectory during adolescence in persons who go on to develop schizophrenia. We focus attention on the findings of GHR research because young, non-psychotic, unmedicated, first-degree relatives of patients represent a particularly valuable population for identifying putative markers of schizophrenia risk associated with early development, preceding psychosis-like symptoms (Cannon et al., 2003). We recognize the speculative nature of our hypotheses, and that there may be other pathways to SDs in schizophrenia that do not involve early brain dysmaturation, or that SDs could arise from alternative mechanisms (e.g., neurodegeneration). Our goal is for these ideas to provide future directions for research into the neurobiology of SDs during the pre-psychosis development of youth at risk for schizophrenia.
2. The neural correlates of “self” in normal development: middle childhood to adolescence
2.1 Behavioral findings
A large literature within developmental psychology suggests that a basic sense of self-other differentiation arises during very early childhood, with some evidence suggesting that a rudimentary sense of physical separateness exists from birth (Fonagy et al., 2002). However, there is growing evidence that the subjective sense of self and the capacity for self-reflection and self-knowledge continue to undergo significant development well into adolescence (Pfeifer and Blakemore, 2012; Sebastian et al., 2008). For example, between middle childhood and adolescence (roughly ages 7–18), behavioral studies suggest that differentiated conceptualizations of the self and other people are progressively built up through the growth of opportunities for social comparison within increasingly diverse interpersonal contexts (Harter, 1999; Harter et al., 1997). Compared to younger children, it has been found that adolescents exhibit a more multidimensional sense of themselves and others. This includes a greater awareness during adolescence of: 1) how the self is perceived by other people (Frankenberger, 2000); and, 2) differing capabilities and attributes within specific social settings (e.g., school versus athletic competition) (Harter and Monsour, 1992; Smollar and Youniss, 1985).
2.2 Structural MRI findings
This development of subjective experience and self-reflective capacity is increasingly linked with ongoing maturation of the neural circuitry of self. Because of evidence (reviewed below) for the protracted development of the neural structures associated with self-reflective processes, it is speculated that the transition to adolescence may mark a period when disturbances of self are increasingly likely to occur, or to become expressed (Blakemore, 2012). Neuroimaging studies focusing on brain structure and white matter (WM) integrity provide growing evidence for proliferation of WM (largely comprised of myelinated axon bundles) in conjunction with decreased gray matter volume ([GM]; an index of cellular and unmyelinated fiber density) in cortical areas recruited during self-processing (e.g., prefrontal and temporal cortices) during the second decade of life (Giedd et al., 1999; Sowell et al., 2002). These changes, at least in part, are believed to result from cellular-level processes, such as myelinization by oligodendrocytes (increased WM) and apoptosis resulting in dendritic pruning (decreased GM), that work in concert to facilitate enhanced regional communication and greater efficiency of neuronal coding during adolescent development (Ernst and Mueller, 2008). Further, there is increasing evidence suggesting that GM reduction occurs according to a consistent temporal pattern beginning first in posterior cortical areas (i.e., parietal cortex) during childhood, and then proceeding to anterior brain regions (e.g., prefrontal cortex (PFC)) during late adolescence and early adulthood (Gogtay et al., 2004; Sowell et al., 2004), with key brain structures involved in the neural circuitry of self (i.e., MPFC) being among the last areas to reach full maturity (Shaw et al., 2008).
2.3 Resting-state fMRI findings
Evidence regarding the functional connectivity of brain areas mediating self-processing in childhood is limited. Resting-state fMRI studies in children and adolescents initially reported that inter-hemispheric (“short-range”) connectivity appeared to decrease, while anterior-posterior (“long-range”) connectivity increased over the course of adolescence (Fair et al., 2009). This has been interpreted as an indication of greater integration and reduced “segregation” of DMN functional connectivity during development (Whitfield-Gabrieli and Ford, 2012). However, recent resting-state fMRI methodological analyses (Power et al., 2012; Van Dijk et al., 2012) have increasingly called attention to the potentially confounding effects of head motion in earlier resting-state studies in children, suggesting that these initial findings should be interpreted with caution.
2.4 FMRI findings
Several fMRI studies involving ToM (i.e., “mentalization,” or the capacity to infer mental states in the self and others) have begun to examine the neural correlates of self-reflection in children and adolescents. These studies have been broadly consistent in showing that during ToM children and adolescents exhibit greater recruitment of MPFC than adults, while adults show increased engagement of lateral temporal cortex (Pfeifer and Blakemore, 2012). One interpretation of these findings is that children and adolescents may employ different cognitive strategies during self-assessment than do adults (Sebastian et al., 2008). Because the function of the lateral temporal cortex has been linked with semantic memory retrieval, it has been suggested that for adults, self-referential processing may be more automatic and involve “stored self-knowledge” (Sebastian et al., 2008). By contrast, children and adolescents may engage in a more effortful, active process of self-inquiry during tasks requiring the retrieval of information about the self and, thus, rely more on MPFC function (Sebastian et al., 2008).
2.5 Summary
Converging evidence from behavioral, structural and functional MRI studies suggest that the neural circuitry of self undergoes important developmental changes during the period between middle childhood and early adulthood. It has been hypothesized that the late maturation of central structures involved in self-reflective processing (e.g., MPFC) may result in an “imbalance” during adolescence between earlier developing mesolimbic structures that mediate reward responsivity and less fully-developed PFC areas involved in self-awareness, planning, and cognitive control (Ernst and Mueller, 2008). Thus, the trajectories of structural and functional brain changes occurring in normal development may contribute to making adolescence a “sensitive period” (Blakemore, 2012) in the development of self -- a time when there is heightened vulnerability to disturbances of self and to the emergence of a broad range of psychopathology, including schizophrenia (Paus et al., 2008).
3. Structural and functional MRI findings in genetic high-risk youth
3.1 The genetic high-risk approach
GHR studies, which focus on the non-psychotic, medication-naïve young first-degree relatives (less than age 30) of persons with schizophrenia provide a valuable method for investigating markers of schizophrenia risk associated with early, premorbid development – i.e., the period preceding the onset of prodromal symptoms, but when neurocognitive and social impairments are well-known to occur (Cannon et al., 2003; Keshavan et al., 2005). According to the GHR model, schizophrenia is hypothesized to result from the combination of multiple genetic and environmental vulnerability factors, each associated with relatively small effects (Stone et al., 2005). Prior to psychosis onset, it is predicted that subclinical neuroanatomical, neurophysiological, and cognitive, or behavioral abnormalities (e.g., altered MPFC structure/function, or SDs) may be reliably identifiable and expressed in non-psychotic, first-degree relatives (Stone et al., 2005).
Few neuroimaging studies have focused on GHR children (i.e., pre-adolescents), and “self” has yet to be directly studied during the early pre-psychosis period. However, several lines of evidence suggest that SDs, and abnormalities of the neural circuitry of self, may occur in youth at risk for schizophrenia. For example, studies of psychopathology in childhood and adolescence have shown that impaired self-reflection (e.g., poor self-other boundary discrimination, or altered sense of self) is found in GHR children (Keshavan et al., 2008), and is also among the earliest reported symptoms in people who later develop schizophrenia (Klosterkotter et al., 2001; Poulton et al., 2000). Further, poor social functioning (Amminger et al., 1999; Tarbox and Pogue-Geile, 2008) and perspective-taking deficits (Schiffman et al., 2004) are found to be significantly greater during childhood in people who later go on to develop schizophrenia. Given that self-referential processing impairments have been linked with deficits of both social function (Lysaker et al., 2010) and other forms of social cognition in schizophrenia (Fisher et al., 2008; Irani et al., 2006), these findings lend further plausibility to the hypothesis that SDs occur in “preschizophrenia children.” Additionally, neuropathological autopsy studies of schizophrenia show microneuroanatomical alterations (e.g., abnormal laminal organization and neuronal orientation) associated with disruptions of neurodevelopmental processes during gestation, or very early development, in PFC, lateral temporal and parietal cortical areas that have been linked with self-processing (Akbarian et al., 1993; Bunney and Bunney, 2000; Torrey, 2007). Similarly, structural MRI findings of aberrant PFC and/or temporal/parietal cortical surface morphology in patients with early-onset (White et al., 2003) and adult-onset schizophrenia (Niznikiewicz et al., 2000; Vogeley et al., 2000) are thought to be indicative of early disturbances of gyrification. Taken together, these findings suggest that the abnormal structure and/or function of brain areas involved in self-processing may represent a marker of schizophrenia risk associated with early, pre-prodromal development. Recently, Thermenos and colleagues have provided a comprehensive review of the neuroimaging literature on GHR youth (Thermenos et al., 2013). Below, we summarize the structural and functional findings with respect to the neural structures associated with the neural circuitry of self.
3.2 Structural MRI findings
Cross-sectional structural MRI analyses in GHR youth have most consistently provided evidence for smaller prefrontal cortical (PFC) structures. This includes less GM volume of the MPFC (Rosso et al., 2010), inferior frontal gyrus (Bhojraj et al., 2011a; Harms et al., 2010; Li et al., 2012), and frontal pole (Rosso et al., 2010) in GHR youth compared to controls. Reduced lateral PFC cortical thickness (Byun et al., 2012; Gogtay et al., 2007; Mattai et al., 2011) and/or PFC gyral surface area (Prasad et al., 2009) in GHR has also been reported. Other brain areas linked with self-processing where GHR have shown structural alterations compared to controls are: 1) ACC (Byun et al., 2012; Diwadkar et al., 2006); 2) lateral temporal cortex, (e.g., smaller bilateral superior temporal gyrus volume (Bhojraj et al., 2009; Gogtay et al., 2007; Gogtay et al., 2003) and reduced temporal cortex surface area (Mattai et al., 2011; Prasad et al., 2010)); 3) parietal cortex (e.g., increased asymmetries and smaller volume of right supramarginal and angular gyri (Bhojraj et al., 2009)). Significant associations between higher levels of attenuated psychotic symptoms and smaller volume, or GM density of PFC (Bhojraj et al., 2011c; Diwadkar et al., 2006; Harris et al., 2004), temporal cortex (Job et al., 2005; Job et al., 2006; Lymer et al., 2006), or parietal cortex (Bhojraj et al., 2011c) in GHR youth and/or young adults have also been reported. Although, structural alterations have been observed in GHR samples with children as young as seven (Prasad et al., 2010), there is insufficient data regarding neural alterations at specific ages, or developmental periods (e.g., middle childhood versus adolescence), to draw firm conclusions about the onset of GM loss in the brain structures involved in the neural circuitry of self among GHR youth (Thermenos et al., 2013).
Consistent with the cross-sectional findings, longitudinal studies have also shown abnormalities of PFC over time in GHR compared to controls; such as, reduced MPFC (obitorfrontal) (Bhojraj et al., 2011c) and overall PFC volume (McIntosh et al., 2011b), in addition to progressive abnormalities of PFC gyrification (Harris et al., 2004) and cortical thickness (Prasad et al., 2010). GM volume decline over time in the temporal lobes bilaterally and right parietal lobe in GHR compared to controls has also been found (Job et al., 2005). Importantly, progressive decline in PFC GM volume has been associated with increasing symptoms in GHR, as well as the development of schizophrenia (Bhojraj et al., 2011c; McIntosh et al., 2011a). Similar associations between greater levels of symptoms and significant reductions in lateral temporal and/or parietal cortical GM volume over time are also reported (Bhojraj et al., 2011c; McIntosh et al., 2011a). Thus, overall there is accumulating evidence for progressive alterations in medial/lateral PFC and lateral temporal cortical structure in GHR individuals, particularly among those individuals who develop symptoms, or transition to schizophrenia (approximately 10%).
3.3 Functional MRI findings
Two resting-state fMRI studies have provided evidence for the abnormal function of brain areas associated with self-processing (e.g., MPFC and PCC) in GHR youth (Jang et al., 2011; Whitfield-Gabrieli et al., 2009). An initial resting-state fMRI study showed MPFC hyperactivity and MPFC-PCC hyperconnectivity in GHR youth compared to controls (Whitfield-Gabrieli et al., 2009). Greater alteration of MPFC functional connectivity in this GHR sample was also correlated with increased levels of psychopathology. A subsequent resting-state fMRI study, also found aberrant functional connectivity in the PFC and PCC in GHR compared to controls, with abnormal PFC connectivity associated with greater levels of symptoms (Jang et al., 2011). However, in this study, GHR individuals showed reduced DMN functional connectivity compared to controls. A third resting-state fMRI study, however, did not find any differences in DMN functional connectivity in GHR (Repovs et al., 2011).
Across task-based fMRI studies, GHR individuals have shown dysfunction of medial and lateral PFC, lateral temporal, and parietal cortical structures. For example, during emotional processing tasks, GHR youth have demonstrated decreased activation of ventral PFC (emotional faces processing) (Diwadkar et al., 2012b) and inferior frontal gyrus and parietal cortex (negative stimuli processing) (Venkatasubramanian et al., 2010) compared to controls. Further, in studies of long-term encoding and language processing GHR have shown decreased activation of the right MPFC/anterior cingulate cortex (sentence completion task) (Whalley et al., 2004), as well as both decreased (Li et al., 2007a) and increased (Li et al., 2007b) inferior frontal gyrus activation during visual discrimination tasks. Also, during a story listening task, compared to controls GHR youth have shown reduced bilateral STG and parietal cortical activation (Rajarethinam et al., 2011). Finally, lateral PFC dysfunction in GHR has been demonstrated across a variety of working memory and attentional/control tasks. The most consistent evidence has been for reduced activation of the right dorsolateral PFC during working memory (Brahmbhatt et al., 2006; Choi et al., 2012; Delawalla et al., 2008; Diwadkar et al., 2012a; Seidman et al., 2006) and attentional/cognitive control (Delawalla et al., 2008) tasks in GHR youth compared to controls. Fewer studies have shown decreased activation involving the left dorsolateral PFC and bilateral parietal cortex (Diwadkar et al., 2012a) and bilateral dorsolateral PFC (Keshavan et al., 2002) during working memory in GHR.
In summary, resting-state and task-based fMRI studies GHR youth provide growing evidence for impaired function of MPFC, dorsolateral PFC, and lateral temporal cortical structures (STS), although the direction of altered activation has often varied both between and within the types of tasks employed. As reviewed by Thermenos et al. in detail, GHR neuroimaging studies have used differing MRI software packages (e.g., Statistical Parametric Mapping (SPM), FreeSrufer), methods to correct for multiple comparisons (e.g., whole brain vs. region-of-interest (ROI)), as well as a diversity of structural MRI morphometric techniques (e.g., voxel-based morphometry and manual parcellation), fMRI tasks (e.g., working memory and semantic encoding), and resting-state fMRI methods (e.g., seed-based, ROI and independent-component analysis (ICA)). At least to some extent, these methodological differences may help explain some of the discrepant/divergent findings within the neuroimaging literature on GHR youth.
4. Discussion
We have proposed that self-disturbances (SDs) may be an early indicator of psychosis risk associated with the abnormal structure and function of the “neural circuitry of self” during the premorbid development of children who go on to develop schizophrenia. As our review of the literature in typically developing children suggests, adolescence may be a period of heightened vulnerability to disturbances of self-experience and/or self-reflective processing (Sebastian et al., 2008). It has been proposed that this vulnerability to identity disturbances in the teenage years is linked to the protracted maturation of the neural structures that underlie self-processing. This could lead to an imbalance during this period between later developing parts of the brain mediating self-regulatory capacities (i.e., medial and lateral PFC structures) and subcortical/limbic areas involved in reward responsivity (Blakemore, 2012; Ernst and Mueller, 2008).
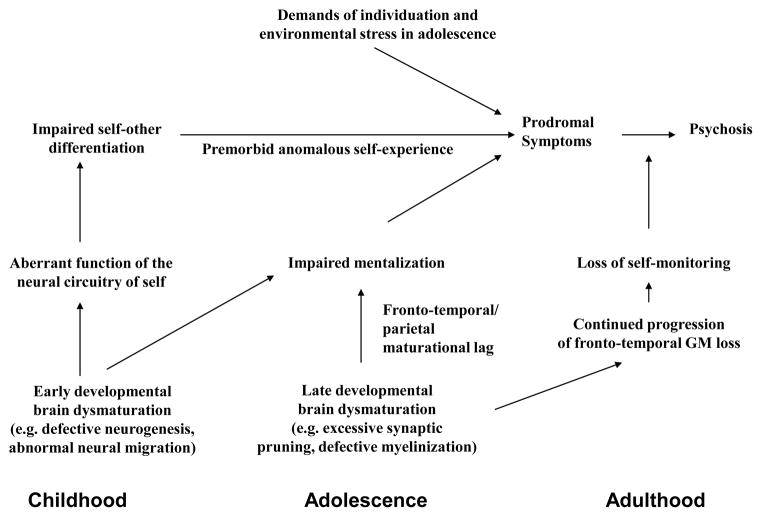
We suggest, however, that, consistent with the “two-hit” neurodevelopmental model of Keshavan and colleagues (Keshavan et al., 1994; Keshavan and Hogarty, 1999), persons at risk for schizophrenia may enter adolescence with an already “vulnerable self” because of brain dysmaturational processes affecting the neural circuitry of self in earlier childhood. Anomalous self-related experiences (e.g., “basic symptoms”) that have been identified in childhood and precede psychosis by many years (Schultze-Lutter, 2009) could be related to subtle, early disturbances affecting the development of an integrated sense of self. In middle childhood, abnormalities of the inferior parietal lobule, for example, could lead to impairments of sensory integration, and subtle alterations of subjectivity and the ability to differentiate self and other. During adolescence, the susceptibility to further disruptions of a more multi-dimensional self, and to impairments of the capacity for mental state understanding (ToM), may be accelerated in at-risk youth. This may stem, in part, from neurodevelopmental alterations involving key brain areas involved in self-processing (e.g., MPFC and lateral temporal cortex) in the context of increasing demands (i.e., environmental stress) associated with individuation and identity formation during adolescence and emerging adulthood. Progressive alterations of the brain structures comprising the neural circuitry of self could then lead to further decline in self-reflection and self-monitoring, contributing to the emergence of prodromal symptoms, followed later by the loss of self-other boundary discrimination and the emergence of psychosis (Figure 1).
Figure 1.
Hypothetical developmental model linking self-disturbances and the emergence of psychosis in schizophrenia. During childhood, alterations of early brain maturational processes lead to the aberrant function of a fronto-temporal-parietal neural circuitry of self and contribute to subtle deficits of self-other differentiation. In adolescence, late developmental brain dysmaturation (excessive synaptic pruning) results in: 1) impairments of mentalization regarding self and other; and 2) anomalous self-experience. With the striving for greater independence and other environmental stressors during teenage development, increased disruption of the sense of self leads to prodromal symptoms. In late-adolescence/early adulthood, progressive fronto-temporal gray matter loss culminates in the breakdown of self-monitoring/reality testing and the emergence of frank psychosis.
Progressive alterations of PFC structure in prodromal individuals who transition to psychosis (Sun et al., 2009), as well as our review of neuroimaging findings in GHR youth, provide some evidence supporting our hypotheses. For example, evidence from structural MRI studies of altered surface morphology of PFC (Prasad et al., 2010) and STS (Bhojraj et al., 2011b) in GHR children and adolescents are believed to indicate the possibility of early abnormalities of neuronal migration and mini-columnar formation occurring during gestation (Keshavan and Bhojraj, 2011; Keshavan and Hogarty, 1999). Additionally, evidence of progressive volume reductions in fronto-temporal-parietal brain regions in GHR youth (Bhojraj et al., 2011c), particularly among those who transition to psychosis (McIntosh et al., 2011a) is consistent with the possibility of aberrant (excessive) synaptic pruning occurring during adolescence (Keshavan et al., 1994). The precise nature of the synaptic pruning abnormality (e.g., hyper- vs. hypo-pruning) in schizophrenia is open to debate (Innocenti et al., 2003). However, neuropathological evidence for reduced neuropil and cortical synaptic density both support a neurodevelopmental model involving excessive synaptic pruning in schizophrenia (Keshavan et al., 1994; McGlashan and Hoffman, 2000). Alternatively, evidence for hypo-pruning in people with schizophrenia is currently lacking.
We recognize the highly speculative nature of our hypotheses, as no studies have directly studied the phenomenology of self-disturbances and its relationship to the structure and function of the neural system of self in youth at risk for schizophrenia. Well-designed studies comparing self-reflection and its neuropsychological correlates in at-risk and typically developing children are crucially needed because establishing the neural correlates of these impairments could have significant implications for early detection and intervention strategies for schizophrenia. This is particularly so because of increasing theory (Brent, 2009; Brent et al., 2013) and empirical evidence (Eack et al., 2009; Lysaker et al., 2007; Salvatore et al., 2012; Subramaniam et al., 2012) suggesting that self-disturbances in people with schizophrenia may be ameliorated by appropriately designed treatments. For example, an increasing number of studies show that psychosocial interventions that focus on self-reflective processes, or perspective-taking (e.g., cognitive training (Eack et al., 2009; Subramaniam et al., 2012) and metacognitive psychotherapy (Lysaker et al., 2007; Salvatore et al., 2012)) may foster the recovery of self-reflective capacities and contribute to improvements in the real world functioning of persons with schizophrenia. For example, Subramaniam and colleagues (Subramaniam et al., 2012) found that patients with schizophrenia who completed a computerized cognitive training program that included ToM exercises showed significant recovery of social function six months after treatment. Importantly, these functional outcomes were also accompanied by significant improvement after treatment in MPFC activation during a reality monitoring fMRI task (Subramaniam et al., 2012). Thus, determining whether at-risk youth show disturbances of self that might be similarly responsive to treatment could make a valuable contribution to early intervention strategies to delay, or preempt, the onset of prodromal symptoms, or the development of schizophrenia.
Acknowledgments
Role of funding source:
None
This project was supported in part by a KL2 Medical Research Investigator Training (MeRIT) award from the Harvard Catalyst and The Harvard Clinical and Translational Science Center, NIH KL2 RR 025757 (BKB), the Commonwealth Research Center of the Massachusetts Department of Mental Health, SCDMH82101008006 (LJS), and by NIMH RO1 64023, 78113, and K02 01180 (MKS).
Footnotes
Contributions:
Benjamin K. Brent wrote the initial draft of this paper. Larry J. Seidman, Heidi W. Thermenos, Daphne J. Holt, and Matcheri S. Keshavan each contributed to the development of the ideas developed in this paper. Matcheri S. Kesahvan managed the overall preparation of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest:
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbarian S, Bunney WE, Jr, Potkin SG, Wigal SB, Hagman JO, Sandman CA, Jones EG. Altered distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase cells in frontal lobe of schizophrenics implies disturbances of cortical development. Archives of general psychiatry. 1993;50(3):169–177. doi: 10.1001/archpsyc.1993.01820150007001. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Pape S, Rock D, Roberts SA, Ott SL, Squires-Wheeler E, Kestenbaum C, Erlenmeyer-Kimling L. Relationship between childhood behavioral disturbance and later schizophrenia in the New York High-Risk Project. The American journal of psychiatry. 1999;156(4):525–530. doi: 10.1176/ajp.156.4.525. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature reviews Neuroscience. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Bedford NJ, Surguladze S, Giampietro V, Brammer MJ, David AS. Self-evaluation in schizophrenia: an fMRI study with implications for the understanding of insight. BMC psychiatry. 2012;12:106. doi: 10.1186/1471-244X-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojraj TS, Francis AN, Montrose DM, Keshavan MS. Grey matter and cognitive deficits in young relatives of schizophrenia patients. NeuroImage. 2011a;54(Suppl 1):S287–292. doi: 10.1016/j.neuroimage.2010.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojraj TS, Francis AN, Rajarethinam R, Eack S, Kulkarni S, Prasad KM, Montrose DM, Dworakowski D, Diwadkar V, Keshavan MS. Verbal fluency deficits and altered lateralization of language brain areas in individuals genetically predisposed to schizophrenia. Schizophrenia research. 2009;115(2–3):202–208. doi: 10.1016/j.schres.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojraj TS, Sweeney JA, Prasad KM, Eack S, Rajarethinam R, Francis AN, Montrose DM, Keshavan MS. Progressive alterations of the auditory association areas in young non-psychotic offspring of schizophrenia patients. Journal of psychiatric research. 2011b;45(2):205–212. doi: 10.1016/j.jpsychires.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojraj TS, Sweeney JA, Prasad KM, Eack SM, Francis AN, Miewald JM, Montrose DM, Keshavan MS. Gray matter loss in young relatives at risk for schizophrenia: relation with prodromal psychopathology. NeuroImage. 2011c;54(Suppl 1):S272–279. doi: 10.1016/j.neuroimage.2010.04.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood NJ, Bentall RP, Ffytche DH, Simmons A, Murray RM, Howard RJ. Persecutory delusions and the determination of self-relevance: an fMRI investigation. Psychological medicine. 2004;34(4):591–596. doi: 10.1017/S0033291703008997. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. Development of the social brain in adolescence. Journal of the Royal Society of Medicine. 2012;105(3):111–116. doi: 10.1258/jrsm.2011.110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Pantelis C. Theory of mind impairments in first-episode psychosis, individuals at ultra-high risk for psychosis and in first-degree relatives of schizophrenia: Systematic review and meta-analysis. Schizophrenia research. 2013;144(1–3):31–36. doi: 10.1016/j.schres.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Bora E, Sehitoglu G, Aslier M, Atabay I, Veznedaroglu B. Theory of mind and unawareness of illness in schizophrenia: is poor insight a mentalizing deficit? European archives of psychiatry and clinical neuroscience. 2007;257(2):104–111. doi: 10.1007/s00406-006-0681-3. [DOI] [PubMed] [Google Scholar]
- Brahmbhatt SB, Haut K, Csernansky JG, Barch DM. Neural correlates of verbal and nonverbal working memory deficits in individuals with schizophrenia and their high-risk siblings. Schizophrenia research. 2006;87(1–3):191–204. doi: 10.1016/j.schres.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Brent B. Mentalization-based psychodynamic psychotherapy for psychosis. Journal of clinical psychology. 2009;65(8):803–814. doi: 10.1002/jclp.20615. [DOI] [PubMed] [Google Scholar]
- Brent BK, Holt DJ, Keshavan MS, Seidman LJ, Fonagy P. Mentalization-based treatment for psychosis: linking an attachment-based model to the psychotherapy for impaired mental state understanding in people with psychotic disorders. The Israel journal of psychiatry and related sciences. 2013 (Accepted) [PubMed] [Google Scholar]
- Brune M, Lissek S, Fuchs N, Witthaus H, Peters S, Nicolas V, Juckel G, Tegenthoff M. An fMRI study of theory of mind in schizophrenic patients with “passivity” symptoms. Neuropsychologia. 2008;46(7):1992–2001. doi: 10.1016/j.neuropsychologia.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Brunet E, Sarfati Y, Hardy-Bayle MC, Decety J. Abnormalities of brain function during a nonverbal theory of mind task in schizophrenia. Neuropsychologia. 2003;41(12):1574–1582. doi: 10.1016/s0028-3932(03)00119-2. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Bunney BG. Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain research Brain research reviews. 2000;31(2–3):138–146. doi: 10.1016/s0165-0173(99)00031-4. [DOI] [PubMed] [Google Scholar]
- Byun MS, Kim JS, Jung WH, Jang JH, Choi JS, Kim SN, Choi CH, Chung CK, An SK, Kwon JS. Regional cortical thinning in subjects with high genetic loading for schizophrenia. Schizophrenia research. 2012;141(2–3):197–203. doi: 10.1016/j.schres.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Bearden CE, Loewy R, Thompson P, Toga AW, Huttunen MO, Keshavan MS, Seidman LJ, Tsuang MT. Early and late neurodevelopmental influences in the prodrome to schizophrenia: contributions of genes, environment, and their interactions. Schizophrenia bulletin. 2003;29(4):653–669. doi: 10.1093/oxfordjournals.schbul.a007037. [DOI] [PubMed] [Google Scholar]
- Cermolacce M, Naudin J, Parnas J. The “minimal self” in psychopathology: reexamining the self-disorders in the schizophrenia spectrum. Consciousness and cognition. 2007;16(3):703–714. doi: 10.1016/j.concog.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Choi JS, Park JY, Jung MH, Jang JH, Kang DH, Jung WH, Han JY, Choi CH, Hong KS, Kwon JS. Phase-specific brain change of spatial working memory processing in genetic and ultra-high risk groups of schizophrenia. Schizophrenia bulletin. 2012;38(6):1189–1199. doi: 10.1093/schbul/sbr038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Cassol H, Phillips C, Balteau E, Salmon E, Van der Linden M. Brains creating stories of selves: the neural basis of autobiographical reasoning. Social cognitive and affective neuroscience. 2013 doi: 10.1093/scan/nst028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delawalla Z, Csernansky JG, Barch DM. Prefrontal cortex function in nonpsychotic siblings of individuals with schizophrenia. Biological psychiatry. 2008;63(5):490–497. doi: 10.1016/j.biopsych.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Montrose DM, Dworakowski D, Sweeney JA, Keshavan MS. Genetically predisposed offspring with schizotypal features: an ultra high-risk group for schizophrenia? Progress in neuro-psychopharmacology & biological psychiatry. 2006;30(2):230–238. doi: 10.1016/j.pnpbp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Pruitt P, Zhang A, Radwan J, Keshavan MS, Murphy E, Rajan U, Zajac-Benitez C. The neural correlates of performance in adolescents at risk for schizophrenia: inefficiently increased cortico-striatal responses measured with fMRI. Journal of psychiatric research. 2012a;46(1):12–21. doi: 10.1016/j.jpsychires.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Wadehra S, Pruitt P, Keshavan MS, Rajan U, Zajac-Benitez C, Eickhoff SB. Disordered corticolimbic interactions during affective processing in children and adolescents at risk for schizophrenia revealed by functional magnetic resonance imaging and dynamic causal modeling. Archives of general psychiatry. 2012b;69(3):231–242. doi: 10.1001/archgenpsychiatry.2011.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack S, Greenwald DP, Hogarty SS, Cooley SJ, DiBarry AL, Montrose DM, Keshavan M. Cognitive enhancement therapy for early-course schizophrenia: effects of a two-year randomized controlled trial. Psychiatric Services. 2009;60:1468–1476. doi: 10.1176/appi.ps.60.11.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Mueller SC. The adolescent brain: insights from functional neuroimaging research. Developmental neurobiology. 2008;68(6):729–743. doi: 10.1002/dneu.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neuroscience and biobehavioral reviews. 2011;35(3):573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Fisher M, McCoy K, Poole JH, Vinogradov S. Self and other in schizophrenia: a cognitive neuroscience perspective. The American journal of psychiatry. 2008;165:1465–1472. doi: 10.1176/appi.ajp.2008.07111806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonagy P, Gergely G, Jurist E, Target M. Affect regulation, mentalization, and the development of the self. New York, New York: Other Press; 2002. [Google Scholar]
- Frankenberger KD. Adolescent egocentrism: a comparison among adolescents and adults. Journal of adolescence. 2000;23(3):343–354. doi: 10.1006/jado.2000.0319. [DOI] [PubMed] [Google Scholar]
- Frith CD, Corcoran R. Exploring ‘theory of mind’ in people with schizophrenia. Psychological medicine. 1996;26(3):521–530. doi: 10.1017/s0033291700035601. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38(1):11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Greenstein D, Lenane M, Clasen L, Sharp W, Gochman P, Butler P, Evans A, Rapoport J. Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia. Archives of general psychiatry. 2007;64(7):772–780. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Sporn A, Clasen LS, Greenstein D, Giedd JN, Lenane M, Gochman PA, Zijdenbos A, Rapoport JL. Structural brain MRI abnormalities in healthy siblings of patients with childhood-onset schizophrenia. The American journal of psychiatry. 2003;160(3):569–571. doi: 10.1176/appi.ajp.160.3.569. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. Journal of cognitive neuroscience. 2004;16(9):1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001a;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature reviews Neuroscience. 2001b;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Harms MP, Wang L, Campanella C, Aldridge K, Moffitt AJ, Kuelper J, Ratnanather JT, Miller MI, Barch DM, Csernansky JG. Structural abnormalities in gyri of the prefrontal cortex in individuals with schizophrenia and their unaffected siblings. The British journal of psychiatry : the journal of mental science. 2010;196(2):150–157. doi: 10.1192/bjp.bp.109.067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JM, Whalley H, Yates S, Miller P, Johnstone EC, Lawrie SM. Abnormal cortical folding in high-risk individuals: a predictor of the development of schizophrenia? Biological psychiatry. 2004;56(3):182–189. doi: 10.1016/j.biopsych.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Harter S. The construction of the self: a developmental perspective. New York, New York: Guilford Press; 1999. [Google Scholar]
- Harter S, Bresnick S, Bouchey HA, Whitesell NR. The development of multiple role-related selves during adolescence. Development and psychopathology. 1997;9(4):835–853. doi: 10.1017/s0954579497001466. [DOI] [PubMed] [Google Scholar]
- Harter S, Monsour A. Developmental analysis of conflict caused among adolescents and adults. Journal of Adolescence. 1992;28:251–260. [Google Scholar]
- Herwig U, Kaffenberger T, Schell C, Jancke L, Bruhl AB. Neural activity associated with self-reflection. BMC neuroscience. 2012;13:52. doi: 10.1186/1471-2202-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Cassidy BS, Andrews-Hanna JR, Lee SM, Coombs G, Goff DC, Gabrieli JD, Moran JM. An anterior-to-posterior shift in midline cortical activity in schizophrenia during self-reflection. Biological psychiatry. 2011;69(5):415–423. doi: 10.1016/j.biopsych.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti GM, Ansermet F, Parnas J. Schizophrenia, neurodevelopment and corpus callosum. Molecular psychiatry. 2003;8(3):261–274. doi: 10.1038/sj.mp.4001205. [DOI] [PubMed] [Google Scholar]
- Irani F, Platek SM, Panyavin IS, Calkins ME, Kohler C, Siegel SJ, Schacter M, Gur RE, Gur RC. Self-face recognition and theory of mind in patients with schizophrenia and first-degree relatives. Schizophrenia research. 2006;88(1–3):151–160. doi: 10.1016/j.schres.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Jang JH, Jung WH, Choi JS, Choi CH, Kang DH, Shin NY, Hong KS, Kwon JS. Reduced prefrontal functional connectivity in the default mode network is related to greater psychopathology in subjects with high genetic loading for schizophrenia. Schizophrenia research. 2011;127(1–3):58–65. doi: 10.1016/j.schres.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Jardri R, Pins D, Lafargue G, Very E, Ameller A, Delmaire C, Thomas P. Increased overlap between the brain areas involved in self-other distinction in schizophrenia. PloS one. 2011;6(3):e17500. doi: 10.1371/journal.pone.0017500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins AC, Mitchell JP. Medial prefrontal cortex subserves diverse forms of self-reflection. Social neuroscience. 2011;6(3):211–218. doi: 10.1080/17470919.2010.507948. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. NeuroImage. 2005;25(4):1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, McIntosh AM, Owens DG, Johnstone EC, Lawrie SM. Grey matter changes can improve the prediction of schizophrenia in subjects at high risk. BMC medicine. 2006;4:29. doi: 10.1186/1741-7015-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LC, Gregg L, Allen P, McGuire PK. Impaired verbal self-monitoring in psychosis: effects of state, trait and diagnosis. Psychological medicine. 2006;36(4):465–474. doi: 10.1017/S0033291705006628. [DOI] [PubMed] [Google Scholar]
- Karbasforoushan H, Woodward ND. Resting-state networks in schizophrenia. Current topics in medicinal chemistry. 2012;12:2404–2414. doi: 10.2174/156802612805289863. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Arnold MC, Bayen UJ, McEvoy JP, Wilson WH. Source-monitoring deficits for self-generated stimuli in schizophrenia: multinomial modeling of data from three sources. Schizophrenia research. 2002;57(1):51–67. doi: 10.1016/s0920-9964(01)00306-1. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of cognitive neuroscience. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Keshavan M, Montrose DM, Rajarethinam R, Diwadkar V, Prasad K, Sweeney JA. Psychopathology among offspring of parents with schizophrenia: relationship to premorbid impairments. Schizophrenia research. 2008;103(1–3):114–120. doi: 10.1016/j.schres.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. Journal of psychiatric research. 1994;28(3):239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Bhojraj T. Gray matter alterations in schizophrenia: are they reversible. In: David AS, Kapur S, McGuffin P, editors. Schizophrenia: The Final Frontier: A Festscrift for Robin M. Murray, Hove. United Kingdom: Psychology Press; 2011. [Google Scholar]
- Keshavan MS, Diwadkar VA, Montrose DM, Rajarethinam R, Sweeney JA. Premorbid indicators and risk for schizophrenia: a selective review and update. Schizophrenia research. 2005;79(1):45–57. doi: 10.1016/j.schres.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Spencer SM, Harenski KA, Luna B, Sweeney JA. A preliminary functional magnetic resonance imaging study in offspring of schizophrenic parents. Progress in neuro-psychopharmacology & biological psychiatry. 2002;26(6):1143–1149. doi: 10.1016/s0278-5846(02)00249-x. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Hogarty GE. Brain maturational processes and delayed onset in schizophrenia. Development and psychopathology. 1999;11(3):525–543. doi: 10.1017/s0954579499002199. [DOI] [PubMed] [Google Scholar]
- Kim HS, Shin NY, Jang JH, Kim E, Shim G, Park HY, Hong KS, Kwon JS. Social cognition and neurocognition as predictors of conversion to psychosis in individuals at ultra-high risk. Schizophrenia research. 2011;130(1–3):170–175. doi: 10.1016/j.schres.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Leube DT. Self-consciousness, self-agency, and schizophrenia. Consciousness and cognition. 2003;12(4):656–669. doi: 10.1016/s1053-8100(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Klosterkotter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Archives of general psychiatry. 2001;58(2):158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- Koren D, Reznik N, Adres M, Scheyer R, Apter A, Steinberg T, Parnas J. Disturbances of basic self and prodromal symptoms among non-psychotic help-seeking adolescents. Psychological medicine. 2012:1–12. doi: 10.1017/S0033291712002322. [DOI] [PubMed] [Google Scholar]
- Koren D, Seidman LJ, Poyurovsky M, Goldsmith M, Viksman P, Zichel S, Klein E. The neuropsychological basis of insight in first-episode schizophrenia: a pilot metacognitive study. Schizophrenia research. 2004;70(2–3):195–202. doi: 10.1016/j.schres.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Langdon R, Michie PT, Ward PB, McConagh N, Catts SV, Coltheart M. Defective self and/or other mentalizing in schizophrenia: a cognitive neuropsychological approach. Cognitive Neuropsychiatry. 1997;2:167–193. doi: 10.1080/135468097396324. [DOI] [PubMed] [Google Scholar]
- Li X, Alapati V, Jackson C, Xia S, Bertisch HC, Branch CA, Delisi LE. Structural abnormalities in language circuits in genetic high-risk subjects and schizophrenia patients. Psychiatry research. 2012;201(3):182–189. doi: 10.1016/j.pscychresns.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Branch CA, Ardekani BA, Bertisch H, Hicks C, DeLisi LE. fMRI study of language activation in schizophrenia, schizoaffective disorder and in individuals genetically at high risk. Schizophrenia research. 2007a;96(1–3):14–24. doi: 10.1016/j.schres.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Branch CA, Bertisch HC, Brown K, Szulc KU, Ardekani BA, DeLisi LE. An fMRI study of language processing in people at high genetic risk for schizophrenia. Schizophrenia research. 2007b;91(1–3):62–72. doi: 10.1016/j.schres.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymer GK, Job DE, William T, Moorhead J, McIntosh AM, Owens DG, Johnstone EC, Lawrie SM. Brain-behaviour relationships in people at high genetic risk of schizophrenia. NeuroImage. 2006;33(1):275–285. doi: 10.1016/j.neuroimage.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Lysaker PH, Buck KD, Ringer J. The recovery of metacognitive capacity in schizophrenia across thritry two months of individual psychotherapy: a case study. Psychotherapy Research. 2007;17:713–720. [Google Scholar]
- Lysaker PH, Carcione A, Dimaggio G, Johannesen JK, Nicolo G, Procacci M, Semerari A. Metacognition amidst narratives of self and illness in schizophrenia: associations with neurocognition, symptoms, insight and quality of life. Acta psychiatrica Scandinavica. 2005;112(1):64–71. doi: 10.1111/j.1600-0447.2005.00514.x. [DOI] [PubMed] [Google Scholar]
- Lysaker PH, Dimaggio G, Carcione A, Procacci M, Buck KD, Davis LW, Nicolo G. Metacognition and schizophrenia: the capacity for self-reflectivity as a predictor for prospective assessments of work performance over six months. Schizophrenia research. 2010;122(1–3):124–130. doi: 10.1016/j.schres.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Mattai AA, Weisinger B, Greenstein D, Stidd R, Clasen L, Miller R, Tossell JW, Rapoport JL, Gogtay N. Normalization of cortical gray matter deficits in nonpsychotic siblings of patients with childhood-onset schizophrenia. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(7):697–704. doi: 10.1016/j.jaac.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Archives of general psychiatry. 2000;57(7):637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Owens DC, Moorhead WJ, Whalley HC, Stanfield AC, Hall J, Johnstone EC, Lawrie S. Longitudinal volume reductions in people at high genetic risk of schizophrenia as they develop psychosis. Biological psychiatry. 2011a;69:953–958. doi: 10.1016/j.biopsych.2010.11.003. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Owens DC, Moorhead WJ, Whalley HC, Stanfield AC, Hall J, Johnstone EC, Lawrie SM. Longitudinal volume reductions in people at high genetic risk of schizophrenia as they develop psychosis. Biological psychiatry. 2011b;69(10):953–958. doi: 10.1016/j.biopsych.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Social psychology as a natural kind. Trends in cognitive sciences. 2009;13(6):246–251. doi: 10.1016/j.tics.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin A, Hamper B. Self-reflection and the inner voice: activation of the left inferior frontal gyrus during perceptual and conceptual self-referential thinking. The open neuroimaging journal. 2012;6:78–89. doi: 10.2174/1874440001206010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ER, Brent BK, Benton M, Pruitt P, Diwadkar V, Rajarethinam RP, Keshavan MS. Differential processing of metacognitive evaluation and the neural circuitry of the self and others in schizophrenia: a pilot study. Schizophrenia research. 2010;116(2–3):252–258. doi: 10.1016/j.schres.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Nelson B, Fornito A, Harrison BJ, Yucel M, Sass LA, Yung AR, Thompson A, Wood SJ, Pantelis C, McGorry PD. A disturbed sense of self in the psychosis prodrome: linking phenomenology and neurobiology. Neuroscience and biobehavioral reviews. 2009;33(6):807–817. doi: 10.1016/j.neubiorev.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Nelson B, Thompson A, Yung AR. Basic self-disturbance predicts psychosis onset in the ultra high risk for psychosis “prodromal” population. Schizophrenia bulletin. 2012;38(6):1277–1287. doi: 10.1093/schbul/sbs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newen A, Vogeley K. Self-representation: searching for a neural signature of self-consciousness. Consciousness and cognition. 2003;12(4):529–543. doi: 10.1016/s1053-8100(03)00080-1. [DOI] [PubMed] [Google Scholar]
- Niznikiewicz M, Donnino R, McCarley RW, Nestor PG, Iosifescu DV, O’Donnell B, Levitt J, Shenton ME. Abnormal angular gyrus asymmetry in schizophrenia. The American journal of psychiatry. 2000;157(3):428–437. doi: 10.1176/appi.ajp.157.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas J. Self and schizophrenia: a phenomenological perspective. Cambridge University Press; Cambridge: 2003. [Google Scholar]
- Parnas J, Handest P, Jansson L, Saebye D. Anomalous subjective experience among first-admitted schizophrenia spectrum patients: empirical investigation. Psychopathology. 2005;38(5):259–267. doi: 10.1159/000088442. [DOI] [PubMed] [Google Scholar]
- Parnas J, Handest P, Saebye D, Jansson L. Anomalies of subjective experience in schizophrenia and psychotic bipolar illness. Acta psychiatrica Scandinavica. 2003;108(2):126–133. doi: 10.1034/j.1600-0447.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- Pauly K, Finkelmeyer A, Schneider F, Habel U. The neural correlates of positive self-evaluation and self-related memory. Social cognitive and affective neuroscience. 2012 doi: 10.1093/scan/nss086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature reviews. Neuroscience. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Blakemore SJ. Adolescent social cognitive and affective neuroscience: past, present, and future. Social cognitive and affective neuroscience. 2012;7(1):1–10. doi: 10.1093/scan/nsr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Archives of general psychiatry. 2000;57(11):1053–1058. doi: 10.1001/archpsyc.57.11.1053. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KM, Goradia D, Eack S, Rajagopalan M, Nutche J, Magge T, Rajarethinam R, Keshavan MS. Cortical surface characteristics among offspring of schizophrenia subjects. Schizophrenia research. 2010;116(2–3):143–151. doi: 10.1016/j.schres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KM, Sanders R, Sweeney J, Montrose D, Diwadkar V, Dworakowski D, Miewald J, Keshavan M. Neurological abnormalities among offspring of persons with schizophrenia: relation to premorbid psychopathology. Schizophrenia research. 2009;108(1–3):163–169. doi: 10.1016/j.schres.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajarethinam R, Venkatesh BK, Peethala R, Phan KL, Keshavan M. Reduced activation of superior temporal gyrus during auditory comprehension in young offspring of patients with schizophrenia. Schizophrenia research. 2011;130(1–3):101–105. doi: 10.1016/j.schres.2011.05.025. [DOI] [PubMed] [Google Scholar]
- Repovs G, Csernansky JG, Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biological psychiatry. 2011;69(10):967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso IM, Makris N, Thermenos HW, Hodge SM, Brown A, Kennedy D, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ. Regional prefrontal cortex gray matter volumes in youth at familial risk for schizophrenia from the Harvard Adolescent High Risk Study. Schizophrenia research. 2010;123(1):15–21. doi: 10.1016/j.schres.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell TA, Rubia K, Bullmore ET, Soni W, Suckling J, Brammer MJ, Simmons A, Williams SC, Sharma T. Exploring the social brain in schizophrenia: left prefrontal underactivation during mental state attribution. The American journal of psychiatry. 2000;157(12):2040–2042. doi: 10.1176/appi.ajp.157.12.2040. [DOI] [PubMed] [Google Scholar]
- Salvatore G, Lysaker PH, Gumley A, Popolo R, Mari J, Dimaggio G. Out of illness experience: metacognition-oriented therapy for promoting self-awareness in individuals with psychosis. American Journal of Psychotherapy. 2012;66:85–106. doi: 10.1176/appi.psychotherapy.2012.66.1.85. [DOI] [PubMed] [Google Scholar]
- Sass LA, Parnas J. Schizophrenia, consciousness, and the self. Schizophrenia bulletin. 2003;29(3):427–444. doi: 10.1093/oxfordjournals.schbul.a007017. [DOI] [PubMed] [Google Scholar]
- Saxe R, Carey S, Kanwisher N. Understanding other minds: linking developmental psychology and functional neuroimaging. Annual review of psychology. 2004;55:87–124. doi: 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. NeuroImage. 2003;19(4):1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Schiffman J, Lam CW, Jiwatram T, Ekstrom M, Sorensen H, Mednick S. Perspective-taking deficits in people with schizophrenia spectrum disorders: a prospective investigation. Psychological medicine. 2004;34(8):1581–1586. doi: 10.1017/s0033291704002703. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. NeuroImage. 2004;22(2):941–947. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Schneider K. Psychopathology. New York, New York: Grune and Stratton; 1959. [Google Scholar]
- Schultze-Lutter F. Subjective symptoms of schizophrenia in research and the clinic: the basic symptom concept. Schizophrenia bulletin. 2009;35(1):5–8. doi: 10.1093/schbul/sbn139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C, Burnett S, Blakemore SJ. Development of the self-concept during adolescence. Trends in cognitive sciences. 2008;12(11):441–446. doi: 10.1016/j.tics.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Thermenos HW, Poldrack RA, Peace NK, Koch JK, Faraone SV, Tsuang MT. Altered brain activation in dorsolateral prefrontal cortex in adolescents and young adults at genetic risk for schizophrenia: an fMRI study of working memory. Schizophrenia research. 2006;85(1–3):58–72. doi: 10.1016/j.schres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smollar J, Youniss J. Adolescent self-concept development. Academic Press; New York, NY: 1985. [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2004;10(4):372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Developmental medicine and child neurology. 2002;44(1):4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophrenia bulletin. 2009;35(3):509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone WS, Faraone SV, Seidman LJ, Olson EA, Tsuang MT. Searching for the liability to schizophrenia: concepts and methods underlying genetic high-risk studies of adolescents. Journal of child and adolescent psychopharmacology. 2005;15(3):403–417. doi: 10.1089/cap.2005.15.403. [DOI] [PubMed] [Google Scholar]
- Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73:842–853. doi: 10.1016/j.neuron.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield JJ, Hassabis D, Maguire EA. Cortical midline involvement in autobiographical memory. NeuroImage. 2009;44(3):1188–1200. doi: 10.1016/j.neuroimage.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Phillips L, Velakoulis D, Yung A, McGorry PD, Wood SJ, van Erp TG, Thompson PM, Toga AW, Cannon TD, Pantelis C. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophrenia research. 2009;108(1–3):85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbox SI, Pogue-Geile MF. Development of social functioning in preschizophrenia children and adolescents: a systematic review. Psychological bulletin. 2008;134(4):561–583. doi: 10.1037/0033-2909.34.4.561. [DOI] [PubMed] [Google Scholar]
- Thermenos HW, Keshavan MS, Juelich RJ, Molokotos E, Whitfield-Gabrieli S, Brent BK, Seidman LJ. Neuroimaging of youg relatives of persons with schizophrenia: a developmental perspective from schizotaxia to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2013 doi: 10.1002/ajmg.b.32170. (Accepted) [DOI] [PubMed] [Google Scholar]
- Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophrenia research. 2007;97(1–3):215–225. doi: 10.1016/j.schres.2007.08.023. [DOI] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neuroscience and biobehavioral reviews. 2010;34(6):935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubramanian G, Puthumana DT, Jayakumar PN, Gangadhar BN. A functional Magnetic Resonance Imaging study of neurohemodynamic abnormalities during emotion processing in subjects at high risk for schizophrenia. Indian journal of psychiatry. 2010;52(4):308–315. doi: 10.4103/0019-5545.74304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Luks TL, Schulman BJ, Simpson GV. Deficit in a neural correlate of reality monitoring in schizophrenia patients. Cerebral cortex (New York, NY: 1991) 2008;18(11):2532–2539. doi: 10.1093/cercor/bhn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley K. Schizophrenia as a disturbance of the self-construct. In: Kircher T, David AS, editors. The Self in Neuroscience and Psychiatry. 2. Cambridge, United Kingdom: Cambridge University Press, Cambridge, UK; 2007. [Google Scholar]
- Vogeley K, Schneider-Axmann T, Pfeiffer U, Tepest R, Bayer TA, Bogerts B, Honer WG, Falkai P. Disturbed gyrification of the prefrontal region in male schizophrenic patients: A morphometric postmortem study. The American journal of psychiatry. 2000;157(1):34–39. doi: 10.1176/ajp.157.1.34. [DOI] [PubMed] [Google Scholar]
- Wang L, Metzak PD, Woodward TS. Aberrant connectivity during self-other source monitoring in schizophrenia. Schizophrenia research. 2011;125(2–3):136–142. doi: 10.1016/j.schres.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Whalley HC, Simonotto E, Flett S, Marshall I, Ebmeier KP, Owens DG, Goddard NH, Johnstone EC, Lawrie SM. fMRI correlates of state and trait effects in subjects at genetically enhanced risk of schizophrenia. Brain : a journal of neurology. 2004;127(Pt 3):478–490. doi: 10.1093/brain/awh070. [DOI] [PubMed] [Google Scholar]
- White T, Andreasen NC, Nopoulos P, Magnotta V. Gyrification abnormalities in childhood- and adolescent-onset schizophrenia. Biological psychiatry. 2003;54(4):418–426. doi: 10.1016/s0006-3223(03)00065-9. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annual review of clinical psychology. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]