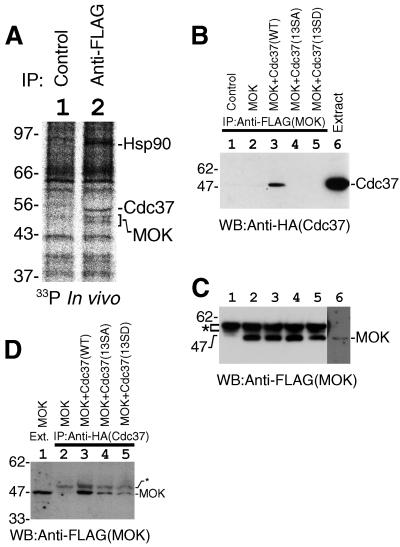
FIG. 4.
The CK2-phosphorylated form of Cdc37 was active in protein kinase binding. (A) Association of the endogenous phosphorylated form of Cdc37 and Hsp90 with MOK in vivo. FLAG-MOK and its associated proteins were isolated by immunoprecipitation from radiolabeled cells. Lane 1, control; lane 2, immunoprecipitation with anti-FLAG antibody. The positions of Hsp90, Cdc37, and MOK are shown. (B) Disruption of the CK2 phosphorylation site of Cdc37 abolished the association of Cdc37 with MOK. FLAG-MOK was expressed in COS7 cells and immunoprecipitated with anti-FLAG antibody. The association of Cdc37 was examined by Western blotting (WB) of the immunoprecipitates. Lane 1, control; lane 2, MOK only; lane 3, MOK plus Cdc37(WT); lane 4, MOK plus Cdc37(13SA); lane 5, MOK plus Cdc37(13SD). Cell extract was included in lane 6 to show the migration position of Cdc37. (C) The equality of immunoprecipitated MOK was confirmed by Western blotting of the immunoprecipitates with anti-FLAG antibody. Lane marks are the same as those for panel B. The position of cross-reacted immunoglobulin heavy-chain bands is shown by an asterisk. The position of MOK was revealed in lane 6 by longer exposure. (D) Ser13 of Cdc37 is important for the stable association with MOK. Cdc37 or its mutants were immunoprecipitated and the association of MOK was examined by Western blotting. In lane 1, extract of cells was included to show the migration position of MOK; lane 2, MOK only; lane 3, MOK plus Cdc37(WT); lane 4, MOK plus Cdc37(13SA); lane 5, MOK plus Cdc37(13SD). Positions of MOK and faint nonspecific bands (asterisk) are indicated on the right.