Table 2.
Optimization of the oxa-Pictet-Spengler reaction with trimethyl orthoformate.a
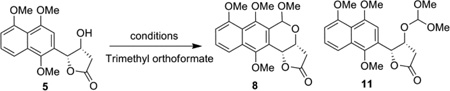 | |||||
|---|---|---|---|---|---|
| entry | Lewis acids |
solvent | temp (ºC) |
time (h) | conversion (%)b |
| 1 | FeCl3 | CH2Cl2 | 0 | 1 | 40(31) |
| 2 | BF3·OEt2 | CH2Cl2 | 0 | 1 | 20 |
| 3 | Fe(OTf)3 | CH2Cl2 | 0 | 2 | 23 |
| 4 | AlCl3 | CH2Cl2 | 0 | 1 | <10 |
| 5 | SnCl4 | CH2Cl2 | 0 | 1 | 45(33) |
| 6 | EtAlCl2 | CH2Cl2 | 0 | 1 | 60(51) |
| 7 | Et2AlCl | CH2Cl2 | 0 | 2 | <10d |
| 8 | EtAlCl2 | CH2Cl2 | −40 | 16 | 45(36) |
| 9 | EtAlCl2 | CH2Cl2 | −20 | 16 | 80(70) |
| 10 | EtAlCl2 | DCE | −20 | 16 | 74(62) |
| 11e | EtAlCl2 | CH2Cl2 | −20 | 16 | 78(65) |
Reaction was performed with 0.2 mmol 5, 0.24 mmol trimethyl orthoformate, and 100 mol % Lewis acid at 0 ºC with 1 h stirring.
Conversion was determined by HPLC analysis. The data in the parentheses are the isolated yields after column chromatography.
dr ratio was determined by the proton NMR of crude products.
formation of the side product 11 in 60% yield.
2 mmol 5 was loaded.
