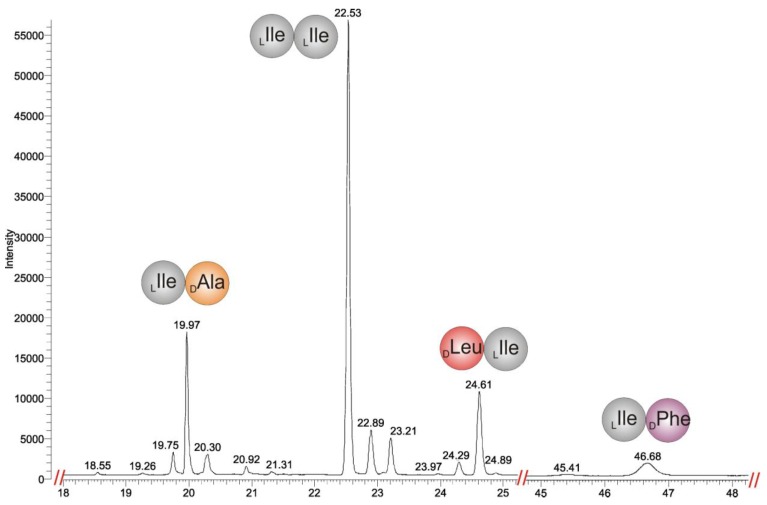
Figure 6.
GC-SIM-Chromatogram on a Chirasil®-(l)-Val column of champacyclin (1a) after N-trifluoroacetyl methyl ester derivatization. Rt (min): 19.97 (L)Ile-(D)Ala, 22.53 (L)Ile-(L)Ile, 24.61 (D)Leu-(L)Ile, 46.68 (L)Ile-(D)Phe.Assignment of stereochemistry with reference dipeptides summarized in Table 1, based on the most abundant peaks in partial hydrolyzate of (1a). Additional peaks correspond to side products from racemization of the dipeptides during partial hydrolysis and derivatization, respectively.