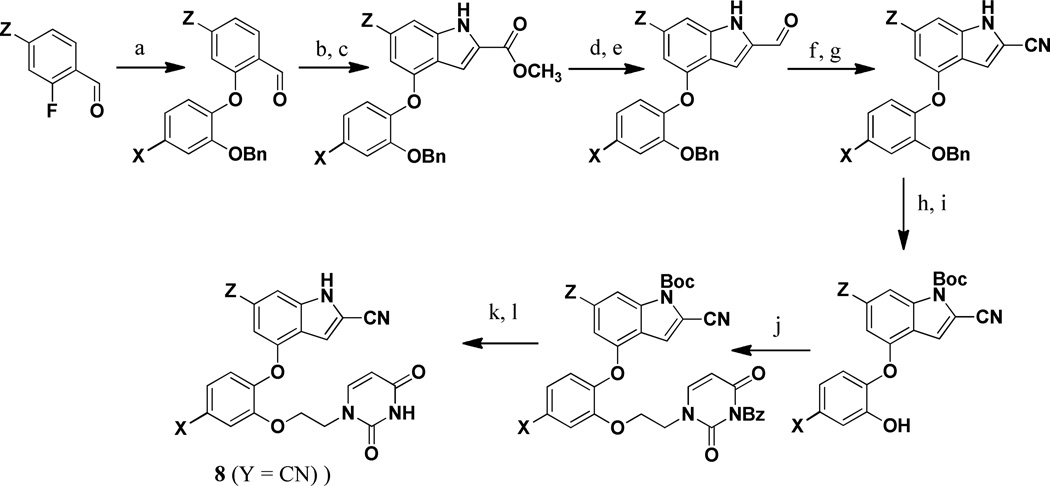
Scheme 1. Synthesis of the 2-Cyanoindoles.
Reagents: (a) K2CO3, DMF, 100–120 °C, 12 h; (b) methyl azidoacetate, −40 °C, NaOMe, MeOH, 12 h; (c) xylene, reflux, 4 h; (d) LAH,THF, 0 °C, 0.5 h; (e) MnO2, DCM, rt, 24 h; (f) NH2 OSO3 H, MeOH, reflux, 0.5 h; (g) Cu(OAc)2, CH3 CN, reflux, 1 h; (h) 4-DMAP, Boc2O, THF, rt, 12 h; (i) H2 /Pd/C, MeOH, rt, 0.5 h; (j) 3-benzoyl,1-bromoethyluracil, K2CO3, DMF, 60 °C, 2 h; (k) TBAF, THF, reflux, 24 h; (l) NH3, MeOH, rt, 12 h.