Abstract
Background
Adenoma detection rate (ADR) has recently been used as a quality measure for screening colonoscopy. We hypothesize that the adenoma detection rate (ADR) will increase with each decade of life after 50 years.
Objective
The aim of this study is to define age-based goals for adenoma detection rate and advanced neoplasia to improve the quality of colonoscopy.
Methods
Utilizing the Clinical Outcomes Research Initiative (CORI) database, patients who underwent screening colonoscopy between 2005-2006 were identified. Pathology of polyp findings was reviewed and the ADR and the prevalence of advanced neoplasia were calculated based on age and gender.
Results
There were 7,756 (44.9%) polypectomies performed on 17,275 patients between 2005-2006. 56.3% (4,363) of these polyps were adenomas or more advanced lesions. The ADR was higher in men than women and increased with age. The ADR in men under age 50 was 24.7 [95% CI 18.2-31.2]; 50-59 years: 27.8 [26.5-29.1]; 60-69 years: 33.6 [31.7-35.4]; 70-79 years: 34.3 [31.5-37.1]; > 80 years: 40.0 [32.9-47.1]. The ADR in women under 50 years old was 12.6 [6.8-18.4]; 50-59 years: 17.0 {15.9-18.1]; 60-69 years: 22.4 {20.8-24.0]; 70-79 years: 26.1 {23.7-28.5]; > 80 years: 26.9 [21.4-32.5].
Limitations
The CORI database offers access to demographic information as well as endoscopy and pathology data but there is limited clinical information about patients in the database.
Conclusion
Adenoma detection rate, and importantly, the rate of advanced neoplasia, increased with each decade of life over 50 and are higher in men than women in each decade of life.
BACKGROUND
Colonoscopy is a widely used screening tool for the detection of colorectal polyps and cancer in adults. The quality of screening colonoscopy is determined predominantly by the identification and removal of adenomas, which has subsequently been shown to reduce the incidence of colorectal cancer 1. Adenoma detection rate (ADR) is defined as the number of patients with adenomas identified per 100 patients screened. This rate has been validated as an independent predictor of the risk of interval colorectal cancer after screening colonoscopy 2. Various studies have also endorsed the use of advanced neoplasia (defined as tubulovillous adenoma, villous adenoma, adenoma > 9mm and carcinoma in situ) as an important endpoint for colonoscopy since these lesions have a substantially increased risk of malignant transformation 3.
Multiple factors can affect the adenoma detection rate including the quality of bowel preparation, withdrawal time and cecal intubation rates 4-6. Current guidelines defined by the American Society for Gastrointestinal Endoscopy (ASGE) and the American College of Gastroenterology (ACG), suggest that in order to obtain quality screening colonoscopy, an endoscopist should achieve an ADR of ≥ 15% in asymptomatic, average risk women over the age of 50 years and ≥ 25% in asymptomatic, average risk men over the age of 50 years7. However, based on several large-scale studies, it is known that the risk and prevalence of adenomas increases with advancing age 7,8.
We hypothesize that the adenoma detection rate and the rate of advanced neoplasia will increase with each decade of life after 50 years old. The aims of this study were to calculate and compare the ADR for men and women in each decade of life over age 50 as well as to compare the rate of advanced neoplasia in both men and women in each decade of life. A secondary aim was to calculate the ADR of different races of patients.
METHODS
Our study examines prospectively collected endoscopic and pathology reports derived from a large U.S. endoscopic database of diverse practice types.
Clinical Outcomes Research Initiative
The Clinical Outcomes Research Initiative (CORI) is a multi-center database of electronic endoscopic reports from 76 adult gastroenterology practices in the United States. The CORI consortium collects demographic and endoscopic data (procedure indication, exam findings, pathology) from diverse practice types in 26 states: 10% academic, 80% community or HMO and 10% Veterans’ Affairs or Military. The CORI database has been validated and the data derived has been shown to be similar to practice patterns of Medicare patients 9. Multiple studies that have utilized CORI data have resulted in peer-reviewed publications 9-11.
Some CORI sites also upload pathology results from their exams into the database. In order to be designated a “colonoscopy pathology site,” at least 75% of all pathology results from colonoscopies must be uploaded into the database. Pathology reports were reviewed from these “pathology sites” and the ADR and rate of advanced neoplasia were calculated by using a denominator of all screening procedures performed.
Patients and Procedures
All patients who underwent average risk screening colonoscopy between 2005-2006 in the aforementioned pathology sites were included in analysis. Exclusion criteria included colonoscopies performed for polyp surveillance, for the evaluation of symptoms and for patients with a family history of colon cancer or polyps. While “exam indication” is a required field in the CORI database, limited patient history introduces possible misclassification bias with potential inclusion of patients undergoing surveillance or diagnostic exams. Data collected from the endoscopic and pathology reports included demographic information, bowel preparation quality, number of polyps detected, polyp location, size and final pathologic diagnosis. Bowel preparation quality was rated on a standard scale: excellent, good, fair/adequate exam, fair/inadequate exam and poor.
Primary and Secondary Endpoints
The primary endpoint of this study was to calculate the adenoma detection rate, defined as the number of patients with an adenoma identified per 100 patients screened, for men and women in each decade of life after 50. An adenoma was defined by pathology results that include tubular adenoma, tubulovillous adenoma and serrated adenoma as well carcinoma in situ, high grade dysplasia and cancer. The detection rate of advanced neoplasia, defined as an adenoma with a diameter of at least 10mm, or with pathology consistent with villous features, high-grade dysplasia, or invasive cancer, was also calculated for men and women in each age group. The ADR of the 50-60 year old group was the reference group for comparison to rates of subsequent decades.
Statistical Analysis
Comparison of categorical data was performed using Pearson's chi-square test of independence and Fisher's Exact test when cell sizes were small. The statistical significance of trend of ADR across each decade was also calculated using the Cochrane-Armitage test for trend. An a priori determined p value of < 0.05 was considered statistically significant. All analyses were performed using SAS software (SAS Institute, Inc., Cary, NC).
RESULTS
Between 2005 and 2006, 17,275 unique patients underwent an average risk screening colonoscopy in our pathology sites of interest. Of these, 7,756 (44.9%) patients had a polypectomy or biopsy of a suspicious lesion performed during their colonoscopy. At least one adenoma (as previously defined) was identified in 4,363 (25.3%) of all patients with polyps.
Table 1 describes the demographics of the men and women in the study cohort. During the study period, 8,795 (50.9%) women and 8,480 (49.1%) men underwent screening colonoscopy. There was no difference in the number of men and women screened in this time period (p < 0.0001). The majority of patients screened were age 50-59 (52.3%). Of all patients screened, only 0.02% were under the age of 50 years; this percentage likely represents average risk patients being screened early due to certain patient preferences but could potentially result from exams being inappropriately classified as screening. White, non-Hispanic patients comprise the majority of the study population (88.2%). The majority of screening colonoscopies in our cohort occurred in the community/HMO site type (88.3%). There were 419 exams excluded because of missing pathology reports. The demographics of those exams missing pathology differed: Black and Asian patients were more likely to have missing pathology reports than white, non-Hispanic patients screened.
Table 1.
Patient demographics.
| FEMALE | MALE | |||
|---|---|---|---|---|
| #screened (% total) | #adenoma (% total) | #screened (% total) | #adenoma (% total) | |
| Total | 8795 | 1765 | 8480 | 2595 |
| Age Group (years) | ||||
| <50 | 127 (1.4) | 16 (0.9) | 170 (2.0) | 42 (1.6) |
| 50-59 | 4576 (52.0) | 778 (44.0) | 4462 (52.6) | 1240 (47.8) |
| 60-69 | 2579 (29.3) | 578 (32.7) | 2528 (29.8) | 849 (32.7) |
| 70-79 | 1268 (14.4) | 331 (18.7) | 1134 (13.4) | 389 (15.0) |
| > 80 | 245 (2.8) | 66 (3.7) | 185 (2.2) | 74 (2.9) |
| Race/Ethnicity | ||||
| White, non-Hispanic | 7746 (88.1) | 1592 (90.0) | 7493 (88.4) | 2342 (90.3) |
| Black, non-Hispanic | 599 (6.8) | 94 (5.3) | 581 (6.9) | 139 (5.4) |
| Asian/Pacific Islander | 248 (2.8) | 51 (2.9) | 184 (2.2) | 55 (2.1) |
| Native American | 35 (0.4) | 5 (0.3) | 48 (0.6) | 13 (0.5) |
| Multi-racial | 23 (0.3) | 3 (0.2) | 10 (0.1) | 3 (0.1) |
| Hispanic | 134 (1.5) | 23 (1.3) | 154 (1.8) | 38 (1.5) |
| Unknown | 10 (0.1) | 1 (0.1) | 9 (0.1) | 3 (0.1) |
| Site Type | ||||
| Community/HMO | 7840 (89.1) | 1619 (91.5) | 7406 (87.3) | 2292 (88.4) |
| Academic | 945 (10.7) | 147 (8.3) | 782 (9.2) | 210 (8.1) |
| VA/Military | 10 (0.1) | 3 (0.2) | 291 (3.4) | 92 (3.5) |
Numbers in parenthesis represent percentages of total.
HMO, Health maintenance organization; VA, Veterans Affairs
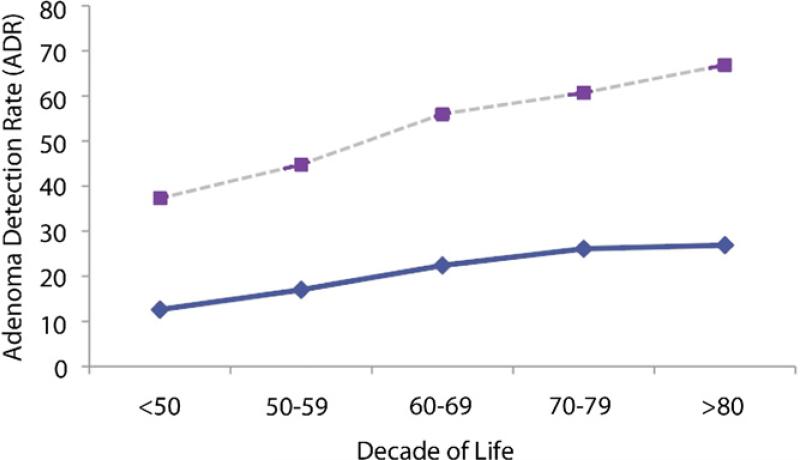
The results of adenoma detection rate based on age and gender are described in Table 2. The 50-59 year old age group had an adenoma detection rate of 17% in women and 27% in men. These rates were used as the reference to determine statistical significance of the ADR of each subsequent decade since they correlate most closely with the current guidelines for ADR. With each subsequent decade of life, there is a statistically significant trend of increasing ADR demonstrated in both men and women. Additionally, figure 1 demonstrates that the ADR for men is higher than that of women in each decade (p value <0.0001).
Table 2.
Adenoma Detection Rate for Men and Women
| MALE | ||||
|---|---|---|---|---|
| Age (years) | ADR | 95% CI | p Value | Trend |
| <50 | 24.7 | 18.4,31.9 | 0.378 | <0.0001 |
| 50-59 | 27.8 | 26.5,29.1 | Reference | |
| 60-69 | 33.6 | 31.7,35.5 | <0.0001 | |
| 70-79 | 34.3 | 31.5,37.2 | <0.0001 | |
| >80 | 40 | 32.9,47.4 | <0.0001 | |
| FEMALE | ||||
|---|---|---|---|---|
| Age (years) | ADR | 95% CI | p Value | Trend |
| <50 | 12.6 | 7.4,19.7 | 0.191 | <0.0001 |
| 50-59 | 17 | 15.9,18.1 | Reference | |
| 60-69 | 22.4 | 20.8,24.1 | <0.0001 | |
| 70-79 | 26.1 | 23.7,28.6 | <0.0001 | |
| >80 | 26.9 | 21.5,32.9 | <0.0001 | |
ADR: Adenoma detection rate; CI: confidence interval
ADR: adenoma detection rate; CI: confidence interval
Figure 1.
ADR. Dashed line represents the ADR in men and the solid line the ADR in women.
Table 3 demonstrates the rate of advanced neoplasia for men and women by age. Advanced neoplasia was identified in 344 (3.91%) women and 527 (6.22%) men in this study cohort; this difference is statistically significant (p <0.0001). The advanced neoplasia rate in men 50-59 years old was 5.09%. In women of the same age group, the advanced neoplasia rate calculated was 3.02%. Once again, with each subsequent decade of life, there is a statistically significant trend of increasing rate of advanced neoplasia demonstrated in both men and women.
Table 3.
Rate of Advanced Neoplasia for Men and Women
| MALE | ||||
|---|---|---|---|---|
| Age | Adv Neo Rate | 95% CI | p value | Trend |
| <50 | 3.53 | 1.31,7.52 | 0.362 | <0.0001 |
| 50-59 | 5.09 | 4.46,5.77 | Reference | |
| 60-69 | 7 | 6.04,8.07 | 0.001 | |
| 70-79 | 8.82 | 7.23,10.62 | <0.001 | |
| >80 | 9.19 | 5.44,14.30 | 0.014 | |
| FEMALE | ||||
|---|---|---|---|---|
| Age | Adv Neo Rate | 95% CI | p value | Trend |
| <50 | 0.79 | 0.02,4.31 | 0.185 | <0.0001 |
| 50-59 | 3.02 | 2.54,3.55 | Reference | |
| 60-69 | 4.54 | 3.77,5.41 | 0.001 | |
| 70-79 | 5.84 | 4.61,7.27 | <0.0001 | |
| >80 | 5.71 | 3.16,9.40 | 0.019 | |
Adv Neo: advanced neoplasia; CI: confidence interval
Adv Neo: advanced neoplasia; CI: confidence interval
The results of adenoma detection rate stratified by age and race for both men and women are recorded in table 4. While there was insufficient power to calculate an ADR for several races, overall, the rate of adenoma detection was higher among whites in all decades when compared to blacks (except in > 80 age group). Finally, table 5 describes adenoma detection rate based on bowel preparation quality. There was no difference in the detection of adenomas if bowel preparation was described by the endoscopist as “excellent” or “good.” However, if bowel preparation was categorized as “fair, exam compromised” or “poor” there were more exams in which no adenoma was detected (p < 0.03 and p < 0.0005).
Table 4.
ADR stratified by age and race
| ADR in MEN | |||||
|---|---|---|---|---|---|
| <50 yo | 50-59 yo | 60-69 yo | 70-79 yo | >80yo | |
| Race | ADR (#) | ADR (#) | ADR (#) | ADR (#) | ADR (#) |
| White | 26.7 (35) | 28.2 (1093) | 34.2 (777) | 35.5 (370) | 38.3 (67) |
| Black | 18.5 (5) | 22.5 (86) | 28.6 (36) | 22.5 (9) | 60 (3) |
| Asian/Pl | 0 (0) | 35.1 (33) | 30.6 (19) | 8.7 (2) | 50 (1) |
| Native American | 0 (0) | 34.8 (8) | 18.8 (3) | 25 (2) | 0 (0) |
| Multi-racial | 0 (0) | 0 (0) | 40 (2) | 100(1) | 0 (0) |
| Hispanic | 28.6 (2) | 23.5 (19) | 25.5 (12) | 22.2 (4) | 100 (1) |
| Unknown | 0 (0) | 33.3 (1) | 0 (0) | 33.3 (1) | 100 (2) |
| ADR in WOMEN | |||||
|---|---|---|---|---|---|
| <50 yo | 50-59 yo | 60-69 yo | 70-79 yo | >80 | |
| Race | ADR (#) | ADR (#) | ADR (#) | ADR (#) | ADR (#) |
| White | 12.4 (13) | 17.5 (684) | 22.4 (523) | 26.4 (308) | 27.5 (64) |
| Black | 21.4 (3) | 13.4 (52) | 18.5 (24) | 23.7 (14) | 14.3 (1) |
| Asian/Pl | 0 (0) | 15.6 (25) | 35.6 (21) | 19.0 (4) | 20 (1) |
| Native American | 0 (0) | 15.8 (3) | 16.7 (2) | 0 (0) | 0 (0) |
| Multi-racial | 0 (0) | 10 (1) | 22.2 (2) | 0 (0) | 0 (0) |
| Hispanic | 0 (0) | 14.5 (12) | 16.7 (6) | 38.5 (5) | 0 (0) |
| Unknown | 0 (0) | 20 (1) | 0 (0) | 0 (0) | 0 (0) |
#: total number of adenoma detected; ADR: Adenoma detection rate; PI: Pacific Islander
All race categories other than Hispanic are non-Hispanic
#: total number of adenoma detected; ADR: Adenoma detection rate; PI: Pacific Islander
All race categories other than Hispanic are non-Hispanic
DISCUSSION
The adenoma detection rate (ADR) is an important quality indicator for colonoscopy7. A recent study from Poland8 has shown a relationship between ADR and subsequent rates of interval cancer. In this study, an ADR less than 20% was associated with a higher risk of interval cancers within several years of the baseline colonoscopy. Variability in ADR has been noted in several studies, and may be due to multiple factors such as exam quality and patient characteristics. ADR may also be dependant on operator technique such as colonoscope withdrawal time 5. Previous studies in Department of Veterans Affairs, military hospitals, and in Poland have shown increasing prevalence of adenomas associated with increasing age 1,8 and related to gender 8,12. The purpose of our study was to determine adenoma detection rates in diverse clinical settings in the United States, and to measure variation based on age and gender of patients undergoing screening examinations.
In this review of a large, multi-center national consortium (CORI), we demonstrate that the adenoma detection rate, as well as the rate of advanced neoplasia, increases for men and women with each decade of life after 50 and is higher in men than women in each decade. While the ADR for both men and women in the 50-59 year old age groups correlates closely with the expected ADR defined by the current guidelines 7, our data demonstrate that there is a statistically significant increase in ADR for both genders with age. These rates were calculated using a denominator of all screening colonoscopies performed in the study group of interest between 2005-2006. We suggest that these trends should be taken into account as gastroenterologists evaluate their own patient populations and outcomes in colonoscopy. For example, if a gastroenterologist's practice is comprised predominantly of men over 60 years old, the ADR for that practice should in fact be closer to 35% than the currently accepted 25%. This case- mix adjustment could further ensure quality performance of screening colonoscopy.
We also find a similar relationship between age and gender in the rates of advanced neoplasia detected at first time screening examinations in both men and women. The finding of advanced neoplasia may be more closely linked to the development of colorectal cancer than the ADR. Future study is needed to determine if detection rates of advanced neoplasia would provide a better surrogate endpoint for exam quality than ADR.
When ADR is stratified by age, gender and race, we find a higher ADR in whites compared to other races in the majority of decades. These data may be limited by the higher proportion of missing pathology reports in both Black and Asian patients. Future study will be necessary to determine if these differences are present on a larger population-wide scale.
One of the key strengths of this study is the representation of diverse community practices, which provide external validity to these results. Nonetheless, these data should be interpreted in the context of the limitations of our study. Clinical and demographic information available in the endoscopic report is entered at the discretion of the endoscopist and therefore may be subject to misclassification bias and clerical error (for example, in assessment of patient race). As previously mentioned, while “exam indication” is a required field in the CORI database, limited patient history introduces possible misclassification bias with potential inclusion of patients undergoing surveillance or diagnostic exams.” We intend to include only screening colonoscopies (ie first exam performed), however patients may have undergone previous exams at centers outside the CORI database and therefore exams designated in CORI as “screening” colonoscopy may in fact be “surveillance” exams. CORI is limited by its lack of correlation with other clinical information such as a medical record. Information such as bowel preparation quality and polyp size (ie ≥ 9mm) is subjective and based on the endoscopist's clinical judgment. Nevertheless, prior studies have demonstrated that endoscopists are able to reliably differentiate between polyps greater than or less than 9mm 13. Importantly, since exams without pathology data were excluded from the calculation of ADR and rates of advanced neoplasia, these calculated rates may in fact underestimate the true ADR and rate of advance neoplasia for each decade however the trends we observed remain significant. Since the 2005-2006 study period, improvements in technology such as high- definition magnification and narrow band imaging have resulted in an increase in ADR as reported in some publications 14,15Nevertheless, the age and sex-related trends we observed are probably still valid.
Despite these limitations, these results are clinically relevant and may be used to augment the current guidelines regarding adenoma detection rate. In summary, these data demonstrates that in a large, diverse, patient population, the rate of both adenoma detection and advanced neoplasia detection is statistically higher with each decade of life after 50 years old in both men and women. In each decade, the ADR and the rate of advanced neoplasia, is higher in men than in women. We believe that case mix adjustment for ADR based on age and gender will enhance the quality of screening colonoscopy.
Acknowledgments
Dr Lieberman received funding from NIDDK UO1 DK57132. In addition, the practice network (CORI) has received support from the following entities to support the infrastructure of the practice-basednetwork: As- traZeneca, Bard International, Pentax USA, ProVation, Endosoft, GIVEN Imaging, and Ethicon. The commercial entities had no involvement in this research.
Footnotes
DISCLOSURE: The following authors disclosed financial relationships relevant to this publication: Dr Lieberman is Executive Director, Dr Eisen is Executive Co-director,and Ms. Holub is an employee of Clinical Outcomes Research Initiative (CORI), a nonprofit organization that receives funding from federal and industry sources. This potential conflict of interest has been reviewed and managed by the OHSU and Veterans Affairs Conflict of Interest in Research Committee. The other authors disclosed no financial relationships relevant to this publication.
References
- 1.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 2.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Prevalence of polyps greater than 9 mm in a consortium of diverse clinical practice settings in the United States. Clin Gastroenterol Hepatol. 2005;3:798–805. doi: 10.1016/s1542-3565(05)00405-2. [DOI] [PubMed] [Google Scholar]
- 4.Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378–384. doi: 10.1016/s0016-5107(04)02776-2. [DOI] [PubMed] [Google Scholar]
- 5.Rex DK. Colonoscopic withdrawal technique is associated with adenoma miss rates. Gastrointest Endosc. 2000;51:33–36. doi: 10.1016/s0016-5107(00)70383-x. [DOI] [PubMed] [Google Scholar]
- 6.Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol. 2006;101:2866–2877. doi: 10.1111/j.1572-0241.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- 7.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873–885. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 8.Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863–1872. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 9.Sonnenberg A, Amorosi SL, Lacey MJ, Lieberman DA. Patterns of endoscopy in the United States: analysis of data from the Centers for Medicare and Medicaid Services and the National Endoscopic Database. Gastrointest Endosc. 2008;67:489–496. doi: 10.1016/j.gie.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 10.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Utilization of colonoscopy in the United States: results from a national consortium. Gastrointest Endosc. 2005;62:875–883. doi: 10.1016/j.gie.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman D, Moravec M, Holub J, Michaels L, Eisen G. Polyp size and advanced histology in patients undergoing colonoscopy screening: implications for CT colonography. Gastroenterology. 2008;135:1100–1105. doi: 10.1053/j.gastro.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoenfeld P, Cash B, Flood A, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med. 2005;352:2061–2068. doi: 10.1056/NEJMoa042990. [DOI] [PubMed] [Google Scholar]
- 13.Schoen RE, Gerber LD, Margulies C. The pathologic measurement of polyp size is preferable to the endoscopic estimate. Gastrointest Endosc. 1997;46:492–496. doi: 10.1016/s0016-5107(97)70002-6. [DOI] [PubMed] [Google Scholar]
- 14.Kahi CJ, Anderson JC, Waxman I, et al. High-definition chromocolonoscopy vs. highdefinition white light colonoscopy for average-risk colorectal cancer screening. Am J Gastroenterol. 2010;105:1301–1307. doi: 10.1038/ajg.2010.51. [DOI] [PubMed] [Google Scholar]
- 15.Inoue T, Murano M, Murano N, et al. Comparative study of conventional colonoscopy and pan-colonic narrow-band imaging system in the detection of neoplastic colonic polyps: a randomized, controlled trial. J Gastroenterol. 2008;43:45–50. doi: 10.1007/s00535-007-2125-x. [DOI] [PubMed] [Google Scholar]