Scheme 1.
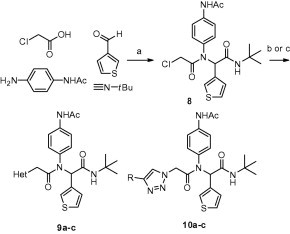
Synthesis of P1 analogs 9a–c and 10a–c. Reagents and conditions: (a) MeOH, 50 °C, 4 h, 95%, (b) (i) NaH, HetNH, DMF, (ii) 9, DMF, 65–80%, (c) (i) NaN3, DMF, 100 °C μwave 30 min, 95%, (ii) acetylene (R = Ph, TMS), DCE, 120 °C 16 h, 85–98%, (iii) R = TMS, TBAF, HOAc, 0 °C–rt, 45%. Final library compounds were purified by UV prep or mass-directed prep HPLC.
