Fig. 3.
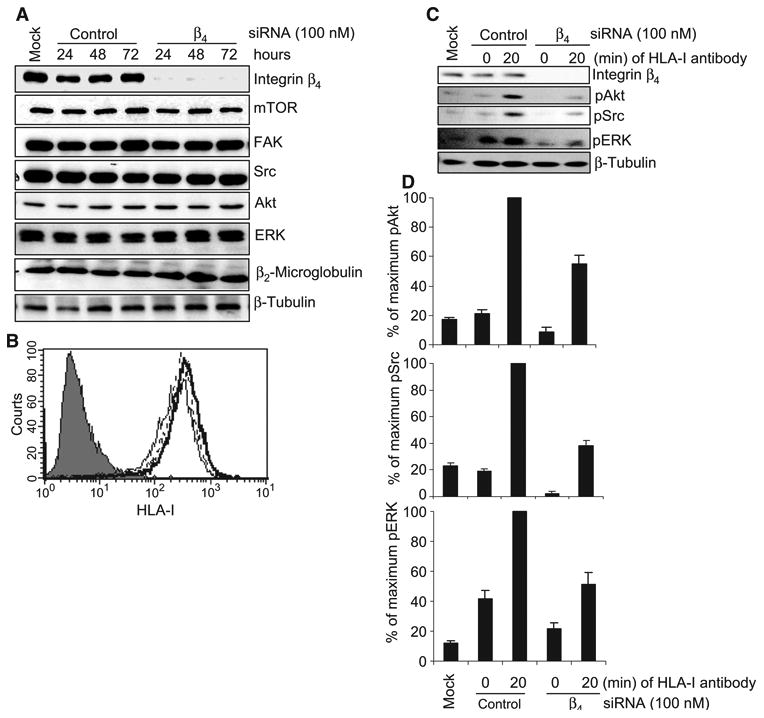
Knockdown of integrin β4 inhibits HLA-I–mediated phosphorylation of target proteins in endothelial cells. (A) Endothelial cells were transfected with 100 nM single duplex siRNA against the integrin β4 subunit (sequence: 5′-GAGAGCAGCUUCCAAAUCA-3′) or with control siRNA and were incubated for 24, 48, or 72 hours. Cells were lysed and subjected to Western blotting analysis with antibodies against integrin β4, mTOR, FAK, Src, Akt, ERK, and β2-microglobulin, with β-tubulin serving as a loading control. (B) Expression of HLA-I on the surface of endothelial cells transfected with siRNAs was analyzed by flow cytometry with the HLA-I–specific antibody W6/32. Negative control cells are shown as a filled peak, whereas the thin, thick, and dotted lines represent mock-transfected cells, cells transfected with integrin β4–specific siRNA, and cells transfected with control siRNA, respectively. (C) Endothelial cells were transfected with integrin β4–specific siRNA or control siRNA (100 nM each). After 48 hours, cells were stimulated with W6/32 (1 μg/ml) for 20 min, lysed, and analyzed by Western blotting with antibodies against pAkt (Ser473), pSrc (Tyr416), and pERK (Thr202/Tyr204). β-Tubulin was detected to determine equal loading. Data shown are representative of three independent experiments. (D) Protein bands shown in (C) were quantified by densitometry and the results are expressed as the mean ± SEM percentage of the maximal extent of protein phosphorylation stimulated by W6/32. Endothelial cells (EC1) were used in these experiments. The data presented in (A) to (C) are representative of at least three independent experiments. (D) Pooled data from three independent experiments.
