Figure 2.
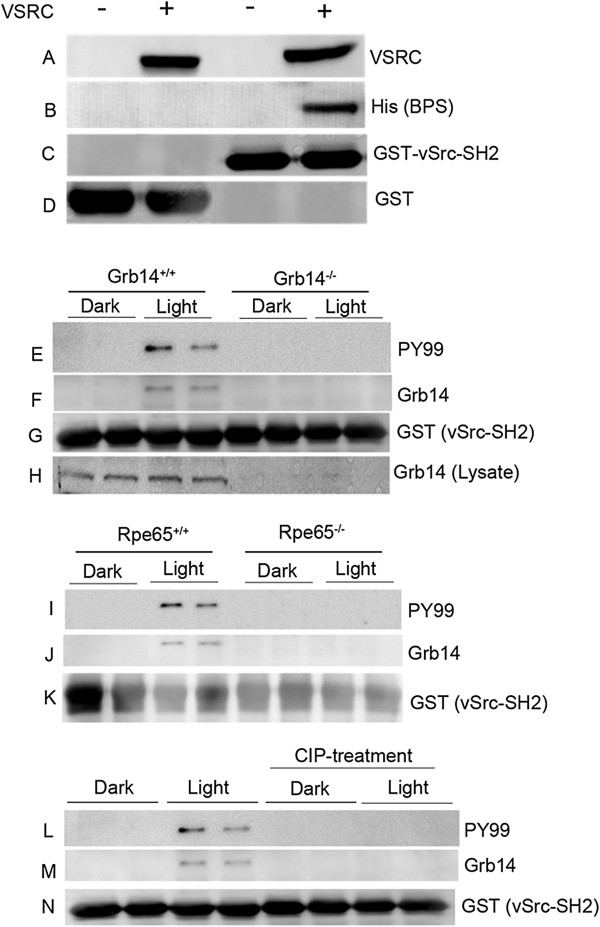
Association of phosphorylated Grb14 with SH2 domain of vSrc in vitro. The His-tagged BPS domain of Grb14 was either expressed or coexpressed with VSRC (A), and the proteins were incubated with GST or GST-vSrc-SH2 fusions followed by a GST pull-down assay. The bound proteins were subjected to immunoblot analysis with the anti-His antibody (B) and reprobed with the anti-GST antibody (C, D). The dark- and light-adapted retinal lysates from wild-type and Grb14-/- mice were subjected to a GST-vSrc-SH2 pull-down assay followed by immunoblotting with anti-PY99 (E), anti-Grb14 (F), and anti-GST (G) antibodies. Wild-type and Grb14-/- mouse retinal lysates were immunoblotted for Grb14 with anti-Grb14 antibody (H). Retinal lysates from dark- and light-adapted wild-type and Rpe65-/- mice were incubated with the GST-vSrc-SH2 fusion protein followed by a GST pull-down assay. The bound proteins were subjected to immunoblotting with anti-PY99 (I) and anti-Grb14 (J) antibodies, and reprobed with anti-GST antibody (K). Retinal lysates from dark- and light-adapted wild-type mice were incubated with the GST-vSrc-SH2 fusion protein followed by GST pull-down assay. One set of the fusion was treated with calf-intestinal phosphatase for 30 min. The fusions were washed and subjected to immunoblotting with anti-PY99 (L) and anti-Grb14 (M) antibodies, and reprobed with anti-GST antibody (N).
