Abstract
Background
This paper describes and discusses the methodology of the Nordic long-term OCD-treatment study (NordLOTS). The purpose of this effectiveness study was to study treatment outcome of CBT, to identify CBT non- or partial responders and to investigate whether an increased number of CBT-sessions or sertraline treatment gives the best outcome; to identify treatment refractory patients and to investigate the outcome of aripiprazole augmentation; to study the outcome over a three year period for each responder including the risk of relapse, and finally to study predictors, moderators and mediators of treatment response.
Methods
Step 1 was an open and uncontrolled clinical trial with CBT, step 2 was a controlled, randomised non-blinded study of CBT non-responders from step 1. Patients were randomized to receive either sertraline plus CBT-support or continued and modified CBT. In step 3 patients who did not respond to either CBT or sertraline were treated with aripiprazole augmentation to sertraline.
Conclusions
This multicenter trial covering three Scandinavian countries is going to be the largest CBT-study for paediatric OCD to date. It is not funded by industry and tries in the short and long-term to answer the question whether further CBT or SSRI is better in CBT non-responders.
Keywords: Study design, Multisite study, Child and adolescents, Obsessive-compulsive disorder, Cognitive behavioural therapy, Stepped care design, Treatment outcome
Background
Obsessive-compulsive disorder (OCD) affects up to 1 in 50 people [1,2], often has its onset in childhood or adolescence [2-5], is associated with severe dysfunction and psychiatric comorbidity in most cases [4,6-9], and often has a chronic course [10]. According to NICE-guidelines the ideal initial treatment in children and adolescents is cognitive behavioural therapy (CBT) alone or CBT and SSRI. CBT seems roughly to have about twice the effect size of SSRI-treatment [11-13], although results between studies have varied a lot. The reasons for this variation have not been clear but may be a consequence of sample characteristics, design issues, and differences with regard to the CBT given.
The clinical effectiveness and the stability of treatment gains after CBT are still to be established [14-16]. In addition, we still do not know to which extent CBT-manuals are transferrable to ordinary clinical settings. Valderhaug et al. showed this to be the case in one study, however this needs to be replicated in studies with more clinics in both specialized OCD clinics and general child psychiatric outpatient clinics [17].
Using an effectiveness study design our specific aims with the NordLOTS study were:
1. to identify and describe a large group of patients with moderate to severe OCD in the Scandinavian countries,
2. to treat patients with CBT with the commonly used number of CBT-sessions with exposure and response prevention (step 1) and to study treatment outcome,
3. to identify CBT non- or partial responders and investigate whether an increased number of CBT-sessions or sertraline treatment gives the best outcome (step 2),
4. to identify treatment refractory patients (to both CBT and sertraline) and investigate the outcome of aripiprazole augmentation (step 3),
5. to study the outcome over a three year period for each responder (in the three steps) including the risk of relapse, and
6. to study predictors, moderators and mediators of treatment response (symptomatic, psychosocial, molecular genetics, neuropsychological factors).
Study design
Stepped care design, with three steps
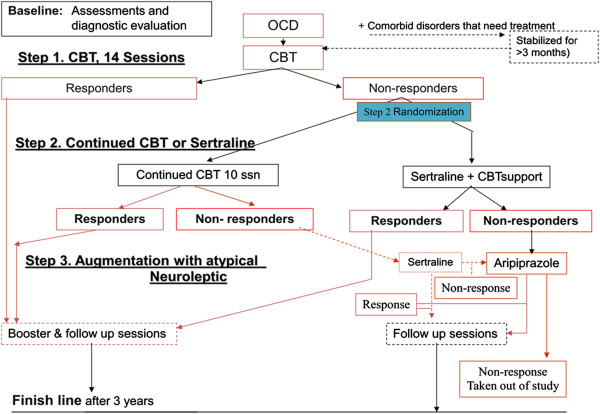
Step 1 was an open and uncontrolled clinical trial in which all patients received cognitive behavioural therapy in the form of exposure and response prevention (E/RP) using 14 treatment sessions. Patients were classified as treatment-responders or non-responders based on Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS), scores of 15 or below or 16 or above respectively post treatment.
Step 2 was a controlled, randomized, non-blinded study of CBT non-responders from step 1. The patients were randomized to receive either sertraline plus CBT-support (in which patients were instructed to practice exposure tasks learned in step 1 outside the study sessions) or continued and modified, individualized CBT.
In step 3 patients who did not respond to either CBT or sertraline were treated with aripiprazole augmentation to sertraline (Figure 1).
Figure 1.
Flowchart.
Randomization was performed using a stratified block method. Randomization sequence in an even ratio between both treatment modalities was done by generating in a two-stage process a block-wise randomization list. The randomization list was stratified according to gender and the presence or absence of tics/Tourette’s syndrome. Evaluation of treatment response was made by an independent evaluator that was not blind to the treatment allocation of each patient.
A multicenter trial
The Scandinavian countries included in this multisite trial (Sweden, Denmark, and Norway) represent a population of approximately 18 million people in total. Although there are some differences in terms of mutual intelligible languages we consider the Scandinavian countries to have mutual cultural background, to a certain extent common history, and therefore are to be considered as a rather homogenous population.
Included in the study trial were clinics which were specialized in OCD-treatment (Aarhus, Denmark, and Gothenburg, Sweden) and further centres in which OCD assessment and treatment were part of a general child and adolescent psychiatric units.
R-BUP in Oslo was the data-, hardware-, and coordination centre with the principal investigator (Ivarsson), Nordic coordinator (Dahl), and research assistant. Twice to thrice yearly meetings by the steering group (all authors) and the executive committee (the study’s initiators (Thomsen, Dahl, and Ivarsson) handled decision making. Yearly meetings at R-BUP with the clinicians and researchers were used to boost compliance.
At each site there was elected a local study coordinator who was responsible for recruitment to the study, the randomization procedure, and data entry.
A study visit was made at each site (once a year) by Tord Ivarsson in order to monitor randomization procedures, the handling of the instruments, assessment procedure, and the data entry into the Confirmit database.
Throughout the study period and during the planning of the study there were more meetings and telephone conferences between the presiding committee or the steering group.
Study period
The study started the inclusion of patients in May 2008 and concluded June 2012.
Population
We aimed at having broad inclusion criteria in order to design a study which was as close to daily clinical practise as possible. Of course, OCD should be the primary disorder and the exclusion criteria were merely those that would make the assessments unreliable or where other treatment needs would have higher priority.
Inclusion criteria
Children and adolescents were included into the NordLOTS on the basis of
1) primary diagnosis of OCD according to the criteria in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision [18],
2) Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) entry score equal to or above 16,
3) ages 7 through 17,
4) patients with attention deficit hyperactivity disorder (ADHD) were included if they had been stable on medication for at least 3 months.
Exclusion criteria
Mental retardation (IQ below 70), disorders with higher treatment priority: autism, primary anorexia nervosa (anorexia in partial remission where OCD had become the residual and primary disorder was permitted), depression with suicidality that demanded CBT, SSRI treatment or inpatient treatment, psychosis, Asperger’s syndrome. However, PDD-NoS was allowed if CGI-score for the PDD was below or equal to 3 and CGI for the PDD-NoS < CGI for the OCD disorder, patients already under treatment for OCD with CBT, SSRI or atypical neuroleptics treatment within six months of study start, the patient or primary caregiver could not speak or understand the language in the country where the study was conducted.
For CBT non-responders who were randomised to sertraline in step 2, two additional exclusion criteria were applied: post pubertal girls with a positive pregnancy test, and post pubertal girls who were sexually active and who did not accept or tolerate adequate contraceptive methods.
Comorbidity
Patients with non-exclusionary comorbid disorder that could still be in need of a special treatment before entering the study, e.g. having ADHD, tics, Tourette’s syndrome or depression, were offered treatment for their comorbid disorder. Patients with treated comorbidity could be included.
Assessment methods
Measures
The following instruments were used as measures for inclusion, predictors and/or measures of treatment outcome (all measures were translated into Norwegian, Swedish, and Danish):
Schedule for affective disorders and schizophrenia for school-age children – present and lifetime version (K-SADS-PL): The K-SADS-PL is a semi-structured diagnostic interview that assesses a range of child psychopathology and demonstrates favourable psychometric properties [19]. K-SADS-PL has shown a good inter-rater reliability of 98% and a 1 to 5 week test-retest kappa of 0.80 for any anxiety disorder diagnosis [19]. Convergent and divergent validity have been documented in a Nordic sample of adolescents [20], moreover, the K-SADS have been used in previous OCD treatment trials [17,21]. Symptoms can be classified as “not present”, “possible”, “in remissions” or “certain”. In this study OCD diagnoses and comorbidity where based on symptoms classified as “certain” only.
Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS): The CY-BOCS is a widely used, clinical-rated, semi-structured interview assessing the severity of OCD symptomatology [22]. The CY-BOCS records symptom categories and evaluates the severity of obsessions and compulsions using10 items, across five dimensions (time occupied by symptoms, interference, distress, resistance, and degree of control over symptoms). The total severity score range from 0 to 40. The CY-BOCS total score in range of 10–18, are considered mild, 19–28 moderate and scores from 29 and above severe [23]. CY-BOCS shows reasonable reliability and validity; with good to excellent inter-rater agreement [24,25]. A high internal consistency, 0.91, 0.68 and 0.84, for obsessions, compulsions and total score respectively, have been shown [26].
Clinical Global Impressions-Severity (CGI-S): Is a clinical rating of symptom severity. Ratings range from 0 (no illness) to 6 (extremely severe). The CGI-S correlates strongly with the CY-BOCS total score in paediatric OCD patients, and is widely used and has been shown to be treatment sensitive [25,27,28].
Clinical Global Impressions-Improvement (CGI–I): The CGI-I is used to assess overall clinical improvement based on symptoms observed and impairment reported using a seven point scale ranging from 0 (very much worse) to 6 (very much improved). The CGI-I scale was dichotomized so patients that received a rating of 5 (much improved) or 6 (very much improved) were collapsed in the analyses. The clinical-rated scale has been used successfully in patients with OCD [27,29].
Children’s Global Assessment Scale (CGAS): is a clinician’s rating on a numeric scale (1–100) of the patient’s overall level of functional strain [30]. The scale has shown good test-retest reliability (r = .62 and r = .76 with psychiatrist and staff respectively). Good inter-rater reliability as well [31]. Furthermore, it has demonstrated both discriminant and concurrent validity [30].
Socioeconomic Status (SES): We used Hollingshead’s two-factor index of social position to classify the socioeconomic position of each family [32]. This two-factor index combines ratings of parental occupation (1–9 scale) and parental education level (1–7 scale). Occupation is given a weight of 5 and education a weight of 3, this generates a summary score. The total scores were transformed into an ordinal scale that ranged between 1 and 5. SES was further dichotomized into two categories, high SES (scores 4–5) and low SES (scores 1–3).
The Child Obsessive-Compulsive Impact Scale (COIS-R): The COIS is a 33-item self-report questionnaire designed to assess the impact of OCD symptoms on the psychosocial functioning of children and adolescent in home, social and academic environment [1]. Both parent and youth versions are available. The patient and parents each rate how much difficulty the child have doing different everyday activities as a result of OCD. Each item is scored on a 4-point Likert scale (0 = not at all, 1 = just a little, 2 = pretty much, and 3 = very much). Both the child and parent versions have shown moderate to high internal consistency, for children α = 0.78 and parents α = 0.92 [1].
Child Behavior Checklist (CBCL): The CBCL is a 113-item parent-report form designed to assess a wide range of child behavioural and emotional problems. Parents rate items on a three-point scale (0 = not true; 1 = somewhat or sometimes true; and 2 = very or often true). This widely used index has established psychometric properties across a variety of clinical and non-clinical populations [33]. The CBCL has shown a mean test-retest reliability between 0.95-1.00 and internal consistency from α = 0.78 to α = 0.97 [33].
Family Accommodation Scale (FAS): The FAS is a 12 item clinician-rated instrument, designed to assess the family’s accommodation to the child’s OCD-symptoms during the previous month [34]. The FAS includes items that measures the extent to which family members provide reassurance or objects needed for compulsions, decreased behavioural expectations of the child, modify family activities or routines, or help the child avoid objects, places or experiences that cause distress. The FAS has demonstrated good psychometric properties including good internal consistency (α = 0.76 to α = 0.80) [34,35], and positive correlation with measures of OCD-symptoms severity [36] and family discord [34].
Screen for Child Anxiety Related Emotional Disorders (SCARED): The SCARED is a psychometrically sound child- and parent-report questionnaire which assesses the presence of DSM-IV anxiety symptoms [37,38]. SCARED total scores were used in these analyses. Scores range from 0 to 82 with higher scores indicating greater impairment and severity. The internal consistency of the SCARED total score was α = 0.94 [39].
The Mood and Feelings Questionnaire (MFQ): The MFQ is based on DSM-III-R criteria for depression and assesses the presence of depressive symptoms by means of 13 items [40]. Scores range from 0 to 26 with higher scores indicating greater impairment and severity. The MFQ has sound psychometric properties [41], and the MFQ total score has shown internal consistency of α = 0.75 to α = 0.78 [42].
Family history of OCD: during baseline assessment parents were asked, in a clinical interview, if they ever have been suffering from OCD. For the present study, a positive family history of OCD means that either the parent(s) and/or the siblings of the identified patient had been diagnosed with OCD.
Parental psychopathology: During baseline assessment parents were asked about psychological symptoms and diagnosed psychiatric problems (yes/no). For the present study, a positive history of parental psychopathology means that a parent(s) of the identified patient had been diagnosed with any psychiatric diagnosis.
Autism Spectrum Screening Questionnaire (ASSQ): The ASSQ was used for a dimensional measure of autism spectrum symptoms [43]. The internal consistency of the ASSQ total score was α = 0.86 [43].
The EAS Temperament Questionnaire (EAS): was used for a dimensional measure of temperament. The questionnaire is a parent report consisting of 20 Likert-scaled items relating to three subscales: emotionality, activity and sociability. The internal consistency of the EAS total score has shown to be α = 0.70 [44].
Questionnaire for Measuring Health-Related Quality of Life in Children and Adolescents (KINDL): was used as self-report questionnaire for children and adolescents as well as a proxy version completed by one of the parents to assess perceived quality of life [45]. The questionnaire consists of 24 items equally distributed into seven subscales. Mean item scores are calculated for all subscales and the total quality of life (QOL) scale, which are transformed to a 0–100 scale, 0 indicates very low and 100 very high QOL. The internal consistency for the children’s self-report total score was α = 0.82 [45].
Five Minute Speech sample (FMSS): The FMSS provides a measurement of parents’ Expressed Emotion (EE) toward their child [46]. The criteria for scoring EE from the FMSS were developed by Magaña et al. (1986) and are based on analyses of the affective quality of the total five minute monologue. Inter-rater reliability is assessed regularly in the laboratory, internal consistency range from α = 0.70 to α = 0.80 [47].
Compliance: During the treatment the clinician assessed the patients’ and the parents’ compliance to the therapy and in therapy. This assessment was done in sessions 2, 7, and 13. Compliance was assessed at a five point scale ranging from 0 (no compliance) to 4 (very good compliance).
Credibility: During treatment, in sessions 2, 7 and 13, the patients and the parents were given a form. They were asked to rate their credibility to the CBT-treatment, if they believed that the therapy would be helpful for them. Credibility was assessed at a five point scale ranging from 0 (no credibility to the therapy) to 4 (very much credibility).
Treatments
CBT step 1
CBT step 1 involved E/RP based on the treatment manuals by March and Mulle as well as an adapted version by Piacentini (unpublished material, 1998), adding more family intervention. The manual was translated from English and adapted to fit Nordic conditions by a group of therapists from the three Nordic countries [48]. Only minor adaptations were necessary, mostly by revising the overall instructions and general descriptions of the main components of the treatment and by putting some more weight on the CBT triangle (the interrelation between thought-emotion-behaviour). Also, our manual put some more stress on the importance of the formulation of exact goals for the child’s play. Nevertheless, the main components from the manuals by March and Mulle, and by Piacentini, were kept unchanged.
An overview of the treatment sessions and the assessment procedures is presented in Table 1.
Table 1.
Content of CBT-sessions and assessments
| Time | Assessment | Assessment instrument | Parents involved in |
|---|---|---|---|
| 0 |
Assessment by independent evaluator |
K-SADS, CBCL, CY-BOCS, CGI, MFQ, ASSQ, COIS, CGAS, FAS, EAS, SCARED-R |
Whole session |
| 1 |
Psycho-education: Model for understanding and treatment |
CGI-I, Compliance |
Whole session |
| 2 |
Externalising of OCD |
CGI-I, |
Whole session |
| 3 |
Cognitive training and further assessment of OCD |
CGI-I, |
30 min |
| Parents: Negative attributions on OCD and the child | |||
| 4 |
Test-exposure and tool box |
CGI-I, |
30 min |
| Parents: Parents’ role, guilt-feeling and self-reproach | |||
| 5 |
E/RP; fight against OCD |
CGI-I, |
30 min |
| Parents: The family’s involvement in OCD | |||
| 6 |
E/RP; get more control over OCD |
CGI-I, |
30 min |
| Parents: The child’s own responsibility for the treatment | |||
| 7 |
E/RP; support the child or the OCD? |
CGI-I, Compliance, CY-BOCS, CGAS, to bring home: MFQ, COIS |
Whole session |
| Joint hour with parents: Repetition of parents’ role, milestones | |||
| 8 |
E/RP; comorbidity and special therapeutic needs. |
CGI-I, |
30 min |
| Parents: Secondary winnings and other obstacles | |||
| 9 |
E/RP; continue the fight against OCD |
CGI-I, |
30 min |
| Parents: Separate OCD from other problems | |||
| 10 |
E/RP; continue the fight against OCD |
CGI-I, |
30 min |
| Parents: Unity and taking care of the family | |||
| 11 |
E/RP; Going through the treatment session |
CGI-I, |
Whole session |
| Parents: Group-gathering – problem solving | |||
| 12 |
E/RP; turning point |
CGI-I, |
30 min |
| Parents: How can parents prevent relapse | |||
| 13 |
E/RP; prevent relapse |
CGI-I, Compliance, CY-BOCS, CGAS. |
30 min |
| Parents: What to do in case of relapse |
CBCL, MFQ, COIS, SCARED-R. |
||
| |
Check that date for independent evaluation is set |
|
|
| 14 | Closing ceremony |
CGI-I, | Whole session |
| Getting together with the parents: Going through the treatment process |
All included patients should ideally have 14 sessions across 14 weeks. Breaks in CBT treatment were minimized and out of 14 sessions at least 10 were performed during at most 4½ months.
In doubtful cases the research group decided whether a patient was to be excluded due to fragmented CBT. Children who were early responders and who wanted to terminate treatment were encouraged to continue to 14 sessions, however, if not possible and fewer sessions had to be allowed (e.g. 1–7 sessions of CBT), ratings at 14 weeks post start should be fixed in time.
Patients who dropped out during step 1 or later were followed in an observational co-study of the NordLOTS using the same follow-up time points.
Step 2 CBT
For step 2, patients were randomized to either SSRI-treatment or CBT in a revised/reformulated version.
Patients randomized to continued CBT received 10 additional treatment sessions over 16 weeks. The same CBT-principles as used in step 1 were used in step 2. However, the therapist was allowed to take an individual approach to treatment based on reassessment of the patient, focusing on factors that may have led to inferior CBT-response. E/RP was adjusted to the problems encountered in step 1. Avoidance was also reassessed, and measures taken to minimize it. In this way the therapist was allowed to adapt CBT-manual to the individual child, situation, family expectations, etc. This was done in order to examine if a more individualized approach could make the CBT-treatment more effective.
Step 2: Medication
The treatment of sertraline included 6 sessions over 16 weeks (week 0, 2, 5, 8, 12, and 16). The pharmacotherapy treatment manual was adapted from the manual used in the POTS study [49]. A starting dose of 25 mg was titrated up to 100 mg over four weeks. Children below 10 years with low weight could be given a lower starting dose. If response was inadequate at a stable dose of 100 mg, after a minimum of 3 weeks, the dose was increased after serum concentration was controlled, if deemed necessary, up to a maximum 200 mg. Response and side effects were controlled at every visit, and dose reduced if necessary. The manual consisted of CBT-support where patients were instructed to practice exposure tasks learned in step 1 outside the study sessions. The main aims of the CBT support was to maintain treatment gains from the first step, to support an active fight against OCD-symptoms upholding a belief that the medication will help, to increase compliance and identify obstacles, and to ensure that medication is accompanied by the same psychological attitude in all cases. However, no new tasks were introduced.
Parents were involved at all medication visits, receiving feed-back about the child’s progress and treatment. While parents were encouraged to praise the child for resisting compulsions, other interventions directed at parents were prohibited during pharmacotherapy (Table 2).
Table 2.
Assessments and dosing schedule in sertraline step 2
| Week | Dose (mg) | Range (mg) | Assessments | Actions |
|---|---|---|---|---|
| |
|
|
|
Check Adverse Events Scale (baseline) (AE), Somatic assessment (SA), Clinical Global Impression (CGI) |
| 0 |
25 × 3 days, then 50 |
25-50 |
CY-BOCS incl. CGI (use CY-BOCS at step 1 session 13 if < 3 weeks, else reassessment in point 10c above), CGAS, blood pressure (BP), pulse, weight, length, side effects (AE) |
AE, SA, CGI, Clinical Global Impression - Improvement (CGI-I) |
| 2 |
75 |
50-75 |
CGI-I, CGI, BP, pulse, weight, length, side effects, treatment credibility |
AE, SA, CGI, CGI-I |
| 3-4 |
100 |
75-100 |
CGI-I, CGI, BP, pulse, weight, length, side effects |
AE, SA, CGI, CGI-I, dose correction based on response |
| 5-7 |
150 |
75-150 |
CGI-I, CGI, BP, pulse, weight, length, side effects, treatment credibility, KINDL (“independent rater”) |
AE, SA, CGI, CGI-I, dose correction on response |
| 8-12 |
200 |
75-200 |
CGI-I, CGI, BP, pulse, weight, length, side effects, treatment credibility |
AE, SA, CGI, CGI-I, dose correction based on response |
| 12-16 | 200 | 75-200 | CY-BOCS incl. CGI/CGI-I, CGAS, Scared-R, MFQ, COIS, FAS, KINDL (“independent rater”), BP, pulse, weight, length, side effects, treatment credibility | AE, SA, CGI, CGI-I, dose correction based on response |
Response to step 2 sertraline treatment
If assessments in session six (at 16 weeks) showed the patient to have a CY-BOCS score of 15 or below, the patient was considered a responder and went to follow-up including sertraline medical checkup and eventually sertraline treatment termination.
If assessment in session six at 16 weeks showed the patient to have a CY-BOCS score of 16 or above this patient was a non-responder and went to step 3 (see later).
Medical checkups and assessments took place every third month. As part of the follow-up (see later) assessments were performed after 6, 12, 24, and 36 months.
Maintenance doses of sertraline
Criteria for lower maintenance dose were checked at every visit: if the patient was very much improved (CGI-I = 6), if the OCD-illness was subclinical or in full remission (CGI-S = 0 or 1), if CY-BOCS scores are ≤ 10 points.
If lowered dose lead to worsened OCD or functioning, the dose was increased to full level again.
Criteria for sertraline termination
If a patient fulfilled termination criteria during medication follow-up, i.e. 6 months of subclinical OCD or full remission, sertraline was lowered with 25% every one to two weeks until a sertraline dose of 25 mg.
Step 3: Aripiprazole augmentation to sertraline in CBT + sertraline non-responders
Patients who were non-responders or partial responders within step 2 of the NordLOTS-study were asked to participate in step 3. Step 3 was based on augmenting sertraline treatment with the antipsychotic drug aripiprazole. Thus, all patients in step 3 were given both sertraline and aripiprazole.
Patients who respond to this regime (i.e. CY-BOCS ≤ 15 and CGI-S ≤ 2, and CGI-I ≥ 5) are followed for the total three year period.
Follow-up
All included patients are being followed-up at 6, 12, 24, and 36 months. They will be assessed with the instruments described in Table 3.
Table 3.
Overview of measurements
| |
|
Step 1 |
Step 2, SSRI and CBT |
Follow up (months) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weeks | Baseline | 7 wks | 13 | Reassess | 8 | 16 | 6 | 12 | 24 | 36 |
| History |
Yes |
|
|
|
|
|
|
|
|
|
| K-SADS [19] |
Yes |
|
|
Possible |
|
|
|
|
|
|
| CY-BOCS [20] |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
| CGI-S/CGI-I [21] |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
| COIS [22] |
Yes |
|
Yes |
Yes |
|
Yes |
Yes |
Yes |
Yes |
Yes |
| CGAS [23] |
Yes |
|
Yes |
Yes |
|
Yes |
Yes |
Yes |
Yes |
Yes |
| CBCL [24] |
Yes |
|
Yes |
|
|
Yes |
Yes |
Yes |
Yes |
Yes |
| YSR [24] |
Yes |
|
Yes |
|
|
|
Yes |
Yes |
Yes |
Yes |
| FAS [25] |
Yes |
|
Yes |
|
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
| FES [14] |
Yes |
|
Yes |
|
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
| PABS [15] |
Yes |
|
Yes |
|
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
| MFQ |
Yes |
|
Yes |
|
|
Yes |
|
|
|
|
| SCARED | Yes | Yes | Yes | |||||||
Genetics
Concomitant study of genetics and heredity
All patients included in the study were asked to participate in a concomitant study on genetic aspects of OCD. All patients filled in a heredity scheme which was used in a semi-structured format quering about relatives that had any of the specified disorders and their severity. This entails taking a saliva sample from the patient and both parents for the study of candidate genes that are part of the glutamate system using a trios design. Treatment response will, we propose, be influenced by genetics as well as by other factors that might interact with genetic factors. Probably, different genes might predict CBT-response as compared to sertraline response.
Quality assurance
All therapists were certified psychotherapists trained in CBT with a special approach to OCD including seminars with local and international speakers training in OCD [1,50]. Before being accepted as a study therapist, each therapist had to have at least two patients with completed CBT-manual.
During the study there was ongoing training in the form of mutual seminars for all study sites and all therapists (at least once a year). Furthermore, the therapists received supervision at each site, and each study patient was discussed at the clinical conferences in the units.
All together, 35 therapists carried out the CBT in step 1. 11 therapists only had two completed patients, six therapists had three patients, and the total range of patients per therapist was 2 to 40. 14 therapists came from Swedish centres, 17 from Norwegian centres and four from the Danish centre.
Treatment fidelity
Each therapist had to fill in a checklist after each session whether he/she had performed E/RP, home work, psychoeducation etc. (different items from session to session according to the treatment manual). Sessions were audio taped so that all phases of CBT (psychoeducation, E/RP and the termination phase) were included.
Three categories of treatment fidelity were evaluated: manual competence, relationship competence and overall evaluation of the session. Scores in each of these categories ranged from 1 = very bad compliance to 4 = very good compliance. Experienced therapists from each country scored recordings of the therapist’s competences using audio tapes and the NordLOTS Treatment Integrity Scale (TIS) for all 14 sessions.
The results of this are described in Torp et al., yet unpublished material.
Reliability of independent evaluator ratings
Each site had independent evaluators. The evaluators from all sites were jointly trained to reliable standard of the CY-BOCS and the Kiddie-SADS through joint interviews, videotaped reviews, and discussion. To maintain and assess reliability between sites 15% of audio taped interviews were chosen for a blind review to assess inter-rater and cross-site reliability. Independent evaluators were used for CY-BOCS at baseline and weeks 7 and 13. Intra-class correlation coefficients of inter-rater agreement were: obsessions ICC = 0.94 (95% CI 0.85-0.97), compulsions ICC = 0.87 (95% CI 0.67-0.93) and total score ICC = 0.92 (95% CI 0.78-0.97).
Statistical aspects
Sample size and randomization
The total number of patients included in step 1 was defined according to the power requirements for step 2.
The primary criteria for entry in step 1 and 2 were moderate to severe symptoms.
Remission in our study was defined as those who had CY-BOCS lower than 16 on the week 13 assessment. Those patients can be regarded to have mild OCD [51,52]. As step 2 was a randomized, controlled trial that included drug treatment we regarded patients with mild OCD (CY-BOCS < 16) to be unsuitable to include. In step 2, we estimated a remission rate of 75% for sertraline plus CBT-support but 50% for the continued CBT without medication. Thus, 58 individuals in each treatment group had to be included to detect such a difference in proportions with an α of 0.05 and β of 0.8 [53]. Seven patients in each treatment condition did not complete the trial and refused any further evaluation on outcome measures.
For analyzing moderators and binary outcome data, multiple imputations were used to replace missing values. This was done with a sequential regression multivariate imputation algorithm with the aid of the IVEware package for SAS [54,55]. The model of imputation included all outcome measures, time in weeks, treatment indicators, stratification variables (sex and tic disorder) and all possible predictors and moderators. A total of 200 data sets were generated to maximize variance [56]. Results that are reported were calculated using the Rubin rules for combining the results of the 200 identical analyses [54].
All randomized patients with a CY-BOCS score more than or equal to 16 before treatment started were included in the analyses according to the principles of intent-to-treat. Analyses were performed with the SAS Statistical Software, version 9.3. The CY-BOCS total score at each assessment point, including step 1, was analyzed with a maximum likelihood estimation of multilevel models [57]. The model included fixed effect days from baseline, introduction of step 2 treatment and days from randomization. Random effects were intercept and days from baseline. The models were fit by using the PROC MIXED in SAS Statistical Software, version 9.3 [58].
Multivariate χ2 test was conducted for testing of between-group differences in the response at week 16. This was done for all the 200 imputations and the results were combined and reported as an F-statistics with the help of the SAS macro combchi [59].
Study monitoring
Independent study monitoring was performed by Dr. Zeiner who was neither involved in the NordLOTS study group nor in the NordLOTS steering group. He was in charge of monitoring step 2 of the trial for signs of 1) safety, 2) effectiveness, and 3) futility. Scientific advisors were Professor John March, Associate Professor Martin Franklin, Professor Bo Larsson, and Professor John Piacentini.
Discussion
Alternative designs to step 1
In this paper we describe the rationale behind the chosen design for the NordLOTS study. Instead of our design we could have chosen CBT versus SSRI, or CBT versus SSRI + CBT. However, both research questions have been addressed in previous studies [28,60,61].
Previous studies have shown that CBT is as sufficient as or even more efficient than SSRI in treating moderate to severe OCD in children and adolescents (Ivarsson et al., yet unpublished material). In the POTS-study it has been shown that CBT alone, sertraline alone and combined treatment are all significantly more efficient than placebo [28]. The POTS-study also documented that combined treatment proved superior to CBT alone and to sertraline alone. However, the remission rate for combined treatment did not differ from that for CBT alone but from sertraline alone and placebo. These studies have led to the general suggestion in most guidelines that children and adolescents with OCD should begin treatment with a combination of CBT plus a SSRI or CBT alone.
The reason for choosing an open step 1 in which all patients received CBT (efficiency study) was to pursue the idea of a stepped care model.
We, therefore, chose CBT as treatment for all enrolled in the study, step 1. Why did we choose 14 sessions? Most of the studies of CBT-outcome in paediatric OCD have employed similar protocols involving weekly treatment over 12–14 weeks [62-64]. Other studies have investigated the effectiveness of 14 weekly sessions over 12 weeks or 18 sessions over 4 weeks without finding any difference. We do not know whether the chosen number of CBT-sessions is indeed the optimal treatment duration. In a meta-analysis of Olatunji et al., 2013, the number of sessions was not related to CBT effect sizes. However, the analysis included all patients no matter whether they were treatment completers or partial- or non-responders [65]. In adults no difference has been found at follow-up between intensive or twice weekly CBT.
The POTS-II study investigated the difference in efficacy between medication, medication plus 7 CBT-sessions, and medication plus 14 CBT-sessions and concluded that dissemination of full CBT-augmentation for paediatric OCD partial responders of SSRI should be an important public health objective [63]. Compared to POTS-II we chose a design which goes in the opposite direction offering CBT as a first step and eventually randomization to SSRI/continued CBT as a step 2, and augmentation to medication therapy as a step 3.
The components of CBT for children have often been poorly specified [66]. E/RP and anxiety management have been used in most studies. It has been shown that E/RP alone (with minimal anxiety management) was sufficient to achieve significant benefit. Other studies have stressed the importance of cognitive techniques [15,28,66]. Few studies have compared CBT in group format and found the same effect of CBT as individualized treatment [14,60]. In few studies the involvement of parents have been tested [14,67]. In this study we chose the protocol by Foa et al. which was translated and adapted to fit Nordic CBT-therapists [48].
Alternatives to step 2 design
In our study non- or partial responders from step 1 were further randomised to a controlled study with a reformulated, intensified CBT or SSRI (+ CBT-support as defined). We chose this design in order to specifically answer the question: does a continued (based on case formulation) CBT help non- or partial responders after 14 sessions of manualized CBT as much as treating with SSRI? This is an often asked question in daily clinical practice.
Why prolonged CBT in step 2? – Some researchers, and many clinicians, have argued for longer treatment in cases of non-response to 14 sessions of CBT [61]. There might be a need for more hours due to the severity of the disorder, and there might be patient characteristics that have led to a delayed CBT-response. Therefore, we have chosen that continued CBT might be a better and more exacting test to pit against SSRI in CBT initial non-responders than would be for instance placebo. The backside of our choice is that the design does not permit to separate out late effects from step 1 CBT.
How important is CBT-support during medical treatment? As we wanted a clinically meaningful treatment arm with SSRI, also including the benefits from the CBT in step 1, we allowed CBT-support - defined as support from the therapist aiming to maintain the gains from the manualized CBT. However, to be able to compare with the CBT step 2-arm no E/RP should be implemented by the pharmacotherapist, as well as any psychotherapy or cognitive behavioural or family intervention during the 30 week study period. Moreover, having all pharmacotherapists give reasonably identical interventions with regard to the OCD-symptoms ought to reduce chance variance due to the pharmacotherapist’s attitude to and knowledge about CBT.
Discussion of representativeness of the sample
The inclusion and exclusion criteria would indicate that all patients with OCD in the study would be fairly representative of patients within the clinics. Our exclusion criteria concern only a small group of OCD-patients and a few patients with higher treatment priorities.
Outcome measures
A cut-off score of 16 or more on the CY-BOCS has been used in previous treatment studies including a number of pharmacological studies [68-70]. In addition, a continuous measurement such as a 30% reduction on the CY-BOCS score is clinically meaningful in order to capture differences in OCD severity and in order to specifically look at subgroups with different responses to treatment within the area of severe, moderate, and mild OCD.
The definition of 10 as a cut-off score for clinical remission has been used in previous studies [28], however, it has recently been questioned by Storch et al. [71]. Response to treatment may be defined differently via a wide range of CY-BOCS percent reductions. Riddle et al. has defined treatment response as a reduction of 25% whereas Geller et al. used a 40% reduction [62]. Tolin aimed at determining the optimal percent reduction cut-offs on the Y-BOCS in 87 adults with OCD after receiving CBT [72]. A Y-BOCS reduction of 30% optimally predicted treatment response, and a Y-BOCS reduction of 40–50% optimally predicted remission. Storch et al. (2010) replicated this analysis in 109 adolescents with OCD. They found that maximally efficient CY-BOCS cut-off was observed at 25% reduction for treatment response, and a 45–50% reduction for symptom remission and that a CY-BOCS score of 14 or below best reflected remission after treatment.
Importance of the study
This is a large study examining the efficiency of CBT in patients in the Scandinavian countries based on a manualized CBT-programme and is to date the largest study in the World. It is not funded by industry and tries in the short and long-term to answer the question whether further CBT or SSRI is better in CBT-non-responders. Knowledge of real world effectiveness is needed for the plans of health organizations, for therapists and doctors who consider choice of treatment, and for the informed patient or parent who wants to participate in treatment planning. Expert clinical guidelines need to be tested empirically to be the basis of these health decisions. The units in the NordLOTS ranges from university based specialized OCD-clinics to unspecialized child and adolescent psychiatric outpatient units. This will make it possible to study the contribution of the type of clinic to treatments success as well.
Relevance to practitioners
First of all, the NordLOTS leads to the development of a CBT and psychopharmacological treatment manual for all Scandinavian countries.
The NordLOTS will hopefully be able to guide practitioners with patients who do not respond to CBT. Furthermore, practitioners are responsible for patients for a long time – in some cases across childhood and adolescence. Most studies only give information on the efficacy of methods across a short period of time. However, the practitioner with a long-term commitment to his/her patient has both to initiate and terminate treatment. The NordLOTS hopefully will be able to handle some but not all of these hurdles. It will give valid information on long-term perspective on treatment up to three years. It will then provide some answers on how to plan treatment with a longitudinal perspective.
Perspectives of the NordLOTS
The studies of psychosocial, symptomatic, and genetic factors on the treatment study will increase our understanding of OCD. In view of the high risk of chronic impairment and suffering in paediatric OCD these aspects need to be highlighted [73,74].
The spreading of the research protocol across the Nordic countries, the therapeutic experience gained from this long study will help us in spreading the expertise in CBT and the adequate use of medication to a high number of outpatient clinics also outside the university based child and adolescent psychiatry.
Ethics
The study was approved by the National ethical Committees in Denmark, Norway, and Sweden. The study was approved by all data authorities in all three countries. Oral and written information was given, and written consent from the parents and patients was received for the RCT-part of the study.
Reporting of results
Results from the CBT-step 1 and predictors for treatment success are going to be published (Torp et al., yet unpublished data). Results from step 2, the randomised trial of continued CBT versus SSRI treatment will be reported (Skarphedinsson et al., yet unpublished data). Data collection for six, 18, 24, and 36 months follow-up studies is ongoing.
EudraCT-number/trial number is 2009-011115-20.
Key points
•The radionale and design of the NordLOTS study is described.
•NordLOTS is the largest multisite, multinational CBT-study for childhood and adolescent OCD describing the effectiveness of a manualized CBT-protocol.
•NordLOTS uses a stepped care design and will attempt to answer the question: What is best for non-responders to 14 sessions of CBT: SSRI-treatment or continued CBT?
•NordLOTS is not sponsored by industry
•NordLOTS will describe the short term as well as the long-term effect of CBT and/or SSRI-treatment after 1, 2, and 3 years.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors have participated in the design of the study and in the collection of data. PHT was in charge of writing this paper on the design and rationale, GS and NCT were in charge of the statistical design. IE, KC, KD, BW, RV, NCT, and KHM all took responsibility in creating the Nordic CBT-manual and also in supervising therapists. All authors read and approved the final manuscript. PHT, KD, TI, and BW were members of the NordLOTS steering group.
Contributor Information
Per Hove Thomsen, Email: Per.hove.thomsen@ps.rm.dk.
Nor C Torp, Email: nor.christian.torp@r-bup.no.
Kitty Dahl, Email: kitty.dahl@r-bup.no.
Karin Christensen, Email: kanchi@rm.dk.
Inger Englyst, Email: jytbre@rm.dk.
Karin H Melin, Email: karin.a.melin@vgregion.se.
Judith B Nissen, Email: judiniss@rm.dk.
Katja A Hybel, Email: katja.hybel@ps.rm.dk.
Robert Valderhaug, Email: robert.valderhaug@ntnu.no.
Bernhard Weidle, Email: b-weidle@online.no.
Gudmundur Skarphedinsson, Email: gudmundur.skarphedinsson@r-bup.no.
Petra Lindheim von Bahr, Email: petra.lindheim-vonbahr@sll.se.
Tord Ivarsson, Email: tord.ivarsson@r-bup.no.
Acknowledgement
We thank Pål Zeiner, John March, John Piacentini, Scott Compton, Martin Franklin, and Bo Larsson for support and advise throughout the study.
Funding
Funding was applied for at each national site so the total study received funding from national funders as well as some central funding. We thank for their contributions that made this study possible.
TrygFonden,
The Danish Council for Strategic Research.
Pulje til styrkelse af psykiatrisk Forskning i Region Midtjylland.
The Center for Child and Adolescent Mental Health, Eastern and Southern Norway (RBUP),
Stiftelsen Clas Groschinskys Minnesfond
Norwegian Research Council, Helse & Rehabilitering, Norge
We are also grateful for all the clinics in our participating countries who donated time for assessments.
References
- Piacentini J, Peris TS, Bergman RL, Chang S, Jaffer M. Functional impairment in childhood OCD: development and psychometrics properties of the Child Obsessive-Compulsive Impact Scale-Revised (COIS-R) J Clin Child Adolesc Psychol. 2007;7:645–653. doi: 10.1080/15374410701662790. [DOI] [PubMed] [Google Scholar]
- Heyman I, Fombonne E, Simmons H, Ford T, Meltzer H, Goodman R. Prevalence of obsessive-compulsive disorder in the British nationwide survey of child mental health. Br J Psychiatry. 2001;7:324–329. doi: 10.1192/bjp.179.4.324. [DOI] [PubMed] [Google Scholar]
- Piacentini J, Bergman RL. Obsessive-compulsive disorder in children. Psychiatr Clin North Am. 2000;7:519–533. doi: 10.1016/S0193-953X(05)70178-7. [DOI] [PubMed] [Google Scholar]
- Valderhaug R, Ivarsson T. Functional impairment in clinical samples of Norwegian ans Swedish children and adolescents with obsessive-compulsive disorder. Eur Child Adolesc Psychiatry. 2005;7:164–173. doi: 10.1007/s00787-005-0456-9. [DOI] [PubMed] [Google Scholar]
- Flament MF, Cohen D. In: Obsessive-Compulsive Disorder. Maj M, Sartorius N, Okasha A, Zohar J, editor. Chichester: John Wiley & Sons, Ltd; 2000. Child and adolescent obsessive-compulsive disorder: a review; pp. 147–183. [Google Scholar]
- Piacentini J, Bergman RL, Keller M, McCracken JT. Functional impairment in children and adolescents with obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 2003;7:S53–S60. doi: 10.1089/104454603322126359. [DOI] [PubMed] [Google Scholar]
- Sørensen CB, Kirkeby L, Thomsen PH. Quality of life with OCD. A self-reported survey among members of the Danish OCD Association. Nord J Psychiatry. 2004;7:231–236. doi: 10.1080/08039480410006287. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Rapoport JL, Leonard HL, Lenane M, Cheslow DL. Obsessive-compulsive disorder in children and adolescents: clinical phenomenology of 70 consecutive cases. Arch Gen Psychiatry. 1989;7:335–341. doi: 10.1001/archpsyc.1989.01810040041007. [DOI] [PubMed] [Google Scholar]
- Ivarsson T, Melin K, Wallin L. Categorical and dimensional aspects of co-morbidity in obsessive-compulsive disorder (OCD) Eur Child Adolesc Psychiatry. 2008;7:20–31. doi: 10.1007/s00787-007-0626-z. [DOI] [PubMed] [Google Scholar]
- Eisen JL, Pinto A, Mancebo MC, Dyck IR, Orlando ME, Rasmusen SA. A 2-year prospective follow-up of the course of obsessive-compulsive disorder. J Clin Psychiatry. 2010;7:1033–1039. doi: 10.4088/JCP.08m04806blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller DA, Biederman J, Stewart SE, Mullin B, Martin A, Spencer T. et al. Which SSRI? A meta-analysis of pharmacotherapy trials in pediatric obsessive-compulsive disorder. Am J Psychiatry. 2003;7:1919–1928. doi: 10.1176/appi.ajp.160.11.1919. [DOI] [PubMed] [Google Scholar]
- March JS, Mulle K, Herbel B. Behavioral psychotherapy for children and adolescents with obsessive-compulsive disorder: an open trial of a new protocol-driven treatment package. JAMA. 1994;7:333–341. doi: 10.1097/00004583-199403000-00006. [DOI] [PubMed] [Google Scholar]
- Watson HJ, Rees CS. Meta-analysis of randomized, controlled treatment trials for pediatric obsessive-compulsive disorder. J Child Psychol Psychiatry. 2008;7:489–498. doi: 10.1111/j.1469-7610.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- Barrett PM, Healy-Farrell L, March JS. Cognitive-behavioral family treatment of childhood obsessive-compulsive disorder: a controlled trial. J Am Acad Child Adolesc Psychiatry. 2004;7:46–62. doi: 10.1097/00004583-200401000-00014. [DOI] [PubMed] [Google Scholar]
- Abramowitz JS, Whiteside SP, Deacon BJ. The effectiveness of treatment for pediatric obsessive-compulsive disorder: a meta-analysis. Behav Ther. 2005;7:55–63. doi: 10.1016/S0005-7894(05)80054-1. [DOI] [Google Scholar]
- Turner CM. Cognitive-behavioural theory and therapy for obsessive-compulsive disorder in children and adolescents: current status and future directions. Clin Psychol Rev. 2006;7:912–938. doi: 10.1016/j.cpr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Valderhaug R, Larsson B, Götestam KG, Piacentini J. An open clinical trial of cognitive-behaviour therapy in children and adolescents with obsessive-compulsive disorder administered in regular outpatient clinics. Behav Res Ther. 2007;7:577–589. doi: 10.1016/j.brat.2006.04.011. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition, text revision (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Rao U, Flynn C, Moreci P. et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;7:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Lauth B, Arnkelsson GB, Magnússon P, Skarphédinsson GÁ, Ferrari P, Pétursson H. Validity of K-SADS-PL (Schedule for Affective Disorders and Schizophrenia for School-Age Children - Present and Lifetime Version) depression diagnoses in an adolescent clinical population. Nord J Psychiatry. 2010;7:409–420. doi: 10.3109/08039481003777484. [DOI] [PubMed] [Google Scholar]
- Freeman JB, Garcia AM, Coyne L, Ale C, Prezeworski A, Himle M. et al. Early childhood ocd: preliminary findings from a family-based cognitive-behavioral approach. J Am Acad Child Adolesc Psychiatry. 2008;7:593–602. doi: 10.1097/CHI.0b013e31816765f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmusen SA, Mazure C, Fleischman RL, Hill CL. et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, Use, and Reliability. Arch Gen Psychiatry. 1989;7:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- March JS, Biederman J, Wolkow R, Safferman A, Mardekian J, Cook EH. et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA. 1998;7:1752–1756. doi: 10.1001/jama.280.20.1752. [DOI] [PubMed] [Google Scholar]
- Gallant J, Storch EA, Merlo LJ, Ricketts ED, Geffken GR, Goodman WK. et al. Convergent and discriminant validity of the Children's Yale-Brown Obsessive Compulsive Scale-Symptom Checklist. J Anxiety Disord. 2008;7:1369–1376. doi: 10.1016/j.janxdis.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Storch EA, Murphy TK, Geffken GR, Soto O, Sajid M, Allen P. et al. Psychometric evaluation of the Children's Yale-Brown Obsessive-Compulsive Scale. Psychiatry Res. 2004;7:91–98. doi: 10.1016/j.psychres.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK. et al. Children's Yale-Brown Obsessive compulsive scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;7:844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- Busner J, Targum SD. The clinical global impressions scale. Applying a research tool in clinical practice. Psychiatry. 2007;7:28–37. [PMC free article] [PubMed] [Google Scholar]
- Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder. The Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;7:1969–1976. doi: 10.1001/jama.292.16.1969. [DOI] [PubMed] [Google Scholar]
- Garvey MA, Perlmutter SJ, Allen AJ, Hamburger S, Lougee L, Leonard HL. et al. A pilot study of penicillin prophylaxis for neuropsychiatric exacerbations triggered by streptococcal infections. Soc Biol Psychiatry. 1999;7:1564–1571. doi: 10.1016/S0006-3223(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H. et al. A Children's Global Assessment Scale (CGAS) Arch Gen Psychiatry. 1983;7:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Green B, Shirk S, Hanze D, Wanstrath J. The children's global assessment scale in clinical practice: an empirical evaluation. J Am Acad Child Adolesc Psychiatry. 1994;7:1158–1164. doi: 10.1097/00004583-199410000-00011. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two factor index of social position. New Haven, CT; 1957. Privately printed. [Google Scholar]
- Achenbach TM. In: The use of psychological testing for treatment planning and outcome assessment. Maurish ME, editor. Hillsdale, NJ: Lawrence Erlbaum Associates; 1994. Child behavior checklist and related instruments; pp. 517–549. [Google Scholar]
- Calvocoressi L, Mazure CM, Kasl SV, Skolnick J, Fisk D, Vegso SJ. et al. Family accommodation of obsessive-compulsive symptoms: instrument development and assessment of family behavior. J Nerv Ment Dis. 1999;7:636–642. doi: 10.1097/00005053-199910000-00008. [DOI] [PubMed] [Google Scholar]
- Geffken GR, Storch EA, Duke DC, Monaco L, Lewin AB, Goodman WK. Hope and coping in family members of patients with obsessive-compulsive disorder. J Anxiety Disord. 2006;7:614–629. doi: 10.1016/j.janxdis.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Storch EA, Geffken GR, Merlo LJ, Jacob ML, Murphy TK, Goodman WK. et al. Family accomodation in pediatric obsessive-compulsive disorder. J Clin Child Adolesc Psychol. 2007;7:207–216. doi: 10.1080/15374410701277929. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiapetta L, Bridge J, Monga S, Baugher M. Psychometric Properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry. 1999;7:1230–1236. doi: 10.1097/00004583-199910000-00011. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J. et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;7:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Muris P, Mayer B, Bartelds E, Tierney S, Bogie N. The revised version of the Screen for Child Anxiety Related Emotional Disorders (SCARED-R): treatment sensitivity in an early intervention trial for childhood anxiety disorders. Br J Clin Psychol. 2001;7:323–336. doi: 10.1348/014466501163724. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Messer SC, Pickles A. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. Int J Methods Psychiatr Res. 1995;7:237–249. [Google Scholar]
- Messer SC, Angold A, Costello EJ, Loeber R, van Kammen W, Stouthamer-Loeber M. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents: factor composition and structure across development. Int J Methods Psychiatr Res. 1995;7:251–262. [Google Scholar]
- Wood A, Kroll L, Moore A, Harrington R. Properties of the mood and feelings questionnaire in adolescent psychiatric outpatients: a research note. J Child Psychol Psychiatry. 1995;7:327–334. doi: 10.1111/j.1469-7610.1995.tb01828.x. [DOI] [PubMed] [Google Scholar]
- Ehlers S, Gillberg C. The epidemiology of Asperger syndrome. A total population study. J Child Psychol Psychiatry. 1993;7:1327–1350. doi: 10.1111/j.1469-7610.1993.tb02094.x. [DOI] [PubMed] [Google Scholar]
- Mathiesen KS, Tambs K. The EAS temperament questionnaire - Factor structure, age trends, reliability, and stability in a Norwegian sample. J Child Psychol Psychiatry. 1999;7:431–439. doi: 10.1111/1469-7610.00460. [DOI] [PubMed] [Google Scholar]
- Bullinger M, Brutt AL, Erhart M, Ravens-Sieberer U. BELLA Study Group. Psychometric properties of the KINDL-R questionnaire: results of the BELLA study. Eur Child Adolesc Psychiatry. 2008;7:125–132. doi: 10.1007/s00787-008-1014-z. [DOI] [PubMed] [Google Scholar]
- Magaña AB, Goldstein MJ, Karno M, Miklowitz DJ, Jenkins J, Falloon IRH. A brief method for assessing expressed emotions in relatives of psychiatric patients. Psychiatry Res. 1986;7:203–212. doi: 10.1016/0165-1781(86)90049-1. [DOI] [PubMed] [Google Scholar]
- Asarnow JR, Goldstein MJ, Tompson MC, Guthrie D. One-Year Outcomes of Depressive Disorders in Child Psychiatric Inpatients: Evaluation of the Prognostic Power of a Brief Measure of Expressed Emotion. J Child Psychol Psychiatry. 1993;7:129–137. doi: 10.1111/j.1469-7610.1993.tb00975.x. [DOI] [PubMed] [Google Scholar]
- Foa EB, Kozak MJ. Mastery of Obsessive-Compulsive Disorder: A Cognitive-Behavioral Approach Therapist Guide. New York, NY: Oxford University Press, Inc.; 1997. [Google Scholar]
- Freeman JB, Choate-Summers ML, Garcia AM, Moore PS, Sapyta JJ, Khanna MS. et al. The pediatric obsessive-compulsive disorder treatment study II: rationale, design and methods. Child Adolesc Psychiatry Ment Health. 2009;7:1–4. doi: 10.1186/1753-2000-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin ME, Kozak MJ, Cashman LA, Coles ME, Rheingold AA, Foa EB. Cognitive-behavioral treatment of pediatric obsessive-compulsive disorder: an open clinical trial. J Am Acad Child Adolesc Psychiatry. 1998;7:412–419. doi: 10.1097/00004583-199804000-00019. [DOI] [PubMed] [Google Scholar]
- Pallanti S, Hollander E, Bienstock C, Koran LM, Leckman JF, Marazziti D. et al. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol. 2002;7:181–191. doi: 10.1017/S1461145702002900. [DOI] [PubMed] [Google Scholar]
- Simpson HB, Huppert JD, Petkova E, Foa EB, Liebowitz MR. Response versus remission in obsessive-compulsive disorder. J Clin Psychiatry. 2006;7:269–276. doi: 10.4088/JCP.v67n0214. [DOI] [PubMed] [Google Scholar]
- Haynes R, Sackett D. Clinical epidemiology: how to do clinical practice research. UK: Lippincott, Williams & Wilkins; 2006. [Google Scholar]
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley Inter-Science: New York, NY; 2004. [Google Scholar]
- Raghunathan TE, Lepkowski JM, van Hoewyk J, Solenberger P. A multivariate technique for multiple imputing missing values using a sequence of regression models. Surv Methodol. 2001;7:85–96. [Google Scholar]
- Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;7:206–213. doi: 10.1007/s11121-007-0070-9. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling change and event occurrence. Oxford: Oxford University Press; 2003. [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Institute, Inc.; 1996. [Google Scholar]
- Allison PD. Macro combchi. 2013. Ref Type: Computer Program.
- Asbahr FR, Castillo AR, Ito LM, Latorre MRDO, Moreira MN, Lotufo-Neto F. Group cognitive-behavioral therapy versus sertraline for the treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2005;7:1128–1136. doi: 10.1097/01.chi.0000177324.40005.6f. [DOI] [PubMed] [Google Scholar]
- de Haan E, Hoogduin KAL, Buitelaar JK, Keijsers GPJ. Behavior therapy versus clomipramine for the treatment of obsessive-compulsive disorder in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1998;7:1022–1029. doi: 10.1097/00004583-199810000-00011. [DOI] [PubMed] [Google Scholar]
- Geller DA, March JS, Walter HJ, Bukstein O, Benson RS, Chrisman A. et al. Practice parameter for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2012;7:98–113. doi: 10.1016/j.jaac.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Franklin ME, Sapyta JJ, Freeman JB, Khanna M, Compton SN, Almirall D. et al. Cognitive behavior therapy augmentation of pharmacotherapy in pediatric obsessive-compulsive disorder: the Pediatric OCD Treatment Study II (POTS II) randomized controlled trial. JAMA. 2011;7:1224–1232. doi: 10.1001/jama.2011.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Kearney RT, Anstey K, von Sanden C, Hunt A. Behavioural and cognitive behavioural therapy for obsessive compulsive disorder in children and adolescents. Cochrane Libr. 20-1-2010. Ref Type: Electronic Citation. [DOI] [PMC free article] [PubMed]
- Olatunji BO, Davis ML, Powers MB, Smits JAJ. COgnitive-behavioral therapy for obsessive-compulsive disorder: a meta-analysis of treatment outcome and moderators. J Psychiatr Res. 2013;7:33–41. doi: 10.1016/j.jpsychires.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Williams TI, Salkovskis PM, Forrester L, Turner S, White H, Allsopp MA. A randomised controlled tiral of cognitive behavioural treatment for obsessive compulsive disorder in children and adolescents. Eur Child Adolesc Psychiatry. 2010;7:449–456. doi: 10.1007/s00787-009-0077-9. [DOI] [PubMed] [Google Scholar]
- Bolton D, Perrin S. Evaluation of exposure with response-prevention for obsessive compulsive disorder in childhood and adolescence. J Behav Ther Exp Psychiatry. 2008;7:11–22. doi: 10.1016/j.jbtep.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Storch EA, Geffken GR, Merlo LJ, Mann G, Duke D, Munson M. et al. Family-based cognitive-behavioral therapy for pediatric obsessive-compulsive disorder: comparison of intensive and weekly approaches. J Am Acad Child Adolesc Psychiatry. 2007;7:469–478. doi: 10.1097/chi.0b013e31803062e7. [DOI] [PubMed] [Google Scholar]
- Riddle MA, Reeve EA, Yaryura-Tobias JA, Yang HM, Claghorn JL, Gaffney G. et al. Fluvoxamine for children and adolescents with obsessive-compulsive disorder: a randomized, controlled, multicenter trial. J Am Acad Child Adolesc Psychiatry. 2001;7:222–229. doi: 10.1097/00004583-200102000-00017. [DOI] [PubMed] [Google Scholar]
- Geller DA, Hoog SL, Heiligenstein JH, Ricardi RK, Tamura R, Kluszynski S. et al. Fluoxetine treatment of obsessive-compulsive disorder in children and adolescents: a placebo-controlled clinical trial. J Am Acad Child Adolesc Psychiatry. 2001;7:773–779. doi: 10.1097/00004583-200107000-00011. [DOI] [PubMed] [Google Scholar]
- Storch EA, Lewin AB, de Nadai AS, Murphy TK. Defining treatment response and remission in obsessive-compulsive disorder: a signal detection analysis of the children's yale-brown obsessive compulsive scale. J Am Acad Child Adolesc Psychiatry. 2010;7:708–717. doi: 10.1016/j.jaac.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Tolin DF. Is cognitive-behavioral therapy more effective than other therapies? A meta-ananlytic review. Clin Psychol Rev. 2010;7:710–720. doi: 10.1016/j.cpr.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Skoog G, Skoog I. A 40-year follow-up of patients with obsessive-compulsive disorder. Arch Gen Psychiatry. 1999;7:121–127. doi: 10.1001/archpsyc.56.2.121. [DOI] [PubMed] [Google Scholar]
- Thomsen PH. Children and adolescents with obsessive-compulsive disorder. A 6–22 year follow-up study of social outcome. Eur Child Adolesc Psychiatry. 1995;7:112–122. doi: 10.1007/BF01977739. [DOI] [PubMed] [Google Scholar]