Abstract
Purpose
This study examined changes in cancer-related knowledge, distress, and decisional conflict from pretest- to post-genetic counseling (GC) in before definitive surgery (BDS) and after definitive surgery (ADS) breast cancer (BC) patients.
Methods
Sociodemographic and clinical characteristics were collected at baseline; primary outcome data were collected before (T1) and after (T2) pretest GC. Within group changes for cancer-related knowledge, distress, and decisional conflict over GT were compared using Wilcoxon signed-rank tests.
Results
Of 103 BC patients, 87 were ADS and 16 were BDS patients. Analyses revealed that both groups reported significant increases in knowledge between T1 and T2 (median change = 4.2, p = .004, and 2.7, p < .001, for BDS and ADS patients, respectively). Overall cancer-related distress showed a downward trend between T1 and T2 for both groups and was significant for BDS patients (p = .041). Reports of BDS patients trended toward overall and subscale-specific increases in decisional conflict, with the exception of the uncertainty which trended downward, but did not reach significance. Overall decisional conflict decreased in ADS patients, approaching marginal significance (p = .056), with significant improvements in informed decision making (median change = -12.6, p < .001; i.e., pretest GC yielded improved knowledge of benefits, risks, and side effects of available options).
Conclusions
These pilot data suggest that pretest GC increases cancer-related knowledge for both BDS and ADS patients, decreases distress in BDS, and improves informed decision making in ADS patients. Future studies with larger sample sizes are needed to replicate these results.
Introduction
In the 10 years after diagnosis, breast cancer (BC) survivors with a BRCA mutation are at substantially elevated contralateral BC (50%)[1, 2] and ovarian cancer (7-13%)[3] risk compared to patients without a BRCA mutation (15-20% new primary BC risk; ∼2% ovarian cancer risk).[4, 5] The National Comprehensive Cancer Network's medical management recommendations vary in intensity and modality for BC survivors with and without a BRCA mutation.[6] Contralateral prophylactic mastectomy reduces contralateral BC risk by ∼ 95%[7, 8] and confers a BC specific and all cause mortality survival advantage for BRCA carriers.[7, 8] Prophylactic bilateral salpingo oophorectomy reduces ovarian cancer risk by ∼ 80%[9, 10] and BC risk by ∼ 50%.[11] A few studies have demonstrated a 50% reduction in contralateral BC incidence for BRCA carriers using tamoxifen, particularly in those without prior prophylactic bilateral salpingo oophorectomy.[12-14] For BRCA carriers who choose surveillance to manage risk, biannual magnetic resonance imaging alternating with mammography is recommended.[15] Given the role of BRCA status in BC treatment and risk management, high-risk BC patients may benefit from BRCA genetic counseling (GC) and genetic testing (GT) at or near the time of initial diagnosis.
The model for providing comprehensive BRCA testing typically begins with an in-person, pretest GC session (See Figure 1).[15-19] Patients meet with a trained health professional to obtain a risk assessment based on personal and family cancer history, receive education about hereditary breast and ovarian cancer (HBOC), and discuss the benefits and limitations of testing. Goals of this initial session are to determine the appropriateness of GT based on the patient's history and risk assessment,[20] as well as to increase HBOC knowledge, aid in psychosocial adjustment, and assist with decision making regarding testing.[21-24]
Figure 1.
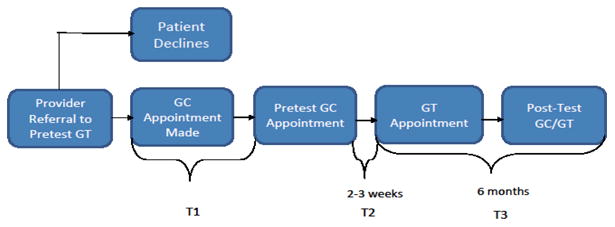
Overview of the genetic counseling and testing process. Current analyses are based on T1 (before pretest, or pre-, GC) and T2 (after pretest, or post-, GC) data.
In general, pretest GC is associated with improvements in cancer-specific knowledge and minimal adverse psychological consequences.[25] However, women affected with cancer may be at increased risk for distress, particularly if the GC and GT process occurs at a time near cancer diagnosis and treatment.[26-28] Yet, little is known about the specific impact of pretest GC on knowledge, psychosocial adjustment, and GT decision making in BC patients before or while undergoing treatment.[29]
A recent study on BC patients approached for GC referral shortly after undergoing surgery demonstrated no increase in short-term psychological burden after GC.[30] What remains unclear, however, is how BC patients who have not undergone definitive surgery respond to pretest GC. For example, before definitive surgery (BDS) patients may feel overwhelmed by the additional risk information and surgical treatment implications presented during GC.[31] Others may be concerned about the emotional impact of genetic vulnerability for themselves or their family at a time when distress is already heightened[32] or perceive limited utility of GC for current treatment decisions.[31, 32] Despite the reality of these perceived barriers, the time surrounding a recent BC diagnosis is, unequivocally, both cognitively and emotionally taxing.[33] Thus, this group may not only perceive but also be at greater risk for distress, difficulty retaining information, and making decisions about GT after pretest GC compared to after definitive surgery (ADS) patients. Conversely, given the focus on risk assessment, discussion of immediate medical management (i.e., surgery), and attention to patients' psychosocial status incorporated into a pretest GC session, it is also possible BDS patients may feel less distressed, experience greater information retention, and have less decisional conflict after pretest GC. To assess BDS and ADS pretest GC outcomes, we conducted a prospective, pilot study to examine the short-term impact of pretest GC on: (a) HBOC knowledge, (b) cancer-related distress, and (c) decisional conflict associated within each group.
Method
Participant Recruitment
As part of a larger, longitudinal study, data were collected: (a) after the pretest GC appointment was scheduled but prior to the GC session (T1), (b) within two to three weeks after participants completed the pretest GC (T2), and (c) six months after completing the T2 assessment (T3). The current report is focused on the short-term impact of pretest GC; therefore, analyses are based on T1 and T2 data (see Figure 1).
Upon Institutional Review Board approval, recruitment began in April 2009 and ended in July 2010. Eligibility criteria included: (a) meeting National Comprehensive Cancer Network cancer genetics referral criteria, (b) ≥ 18 years of age; (c) confirmed personal BC diagnosis based on medical records review; (d) no previous participation in GC and/or testing for HBOC; (e) capable of speaking and reading standard English; (f) having a mailing address and working telephone number; and (g) having a GC appointment scheduled at Moffitt Cancer Center.
The study genetic counselor reviewed the GC appointment schedule weekly for women meeting study eligibility criteria. Eligible patients were mailed a study packet including an introductory letter with a toll-free number to opt out of further contact by the study team, the T1 survey, two consent forms, and a pre-addressed envelope. Approximately one week from the mailing date, patients who did not opt out were contacted via telephone to confirm receipt of study materials and to answer any questions they may have. For those not reachable by telephone prior to their scheduled GC appointment, the study coordinator met briefly with patients after their GC session to determine whether the T1 survey was complete. Those who did not complete the T1 survey before attending their pretest GC session were considered decliners.
For the first six weeks of study recruitment, patients who failed to attend their pretest GC appointment were considered ineligible. However, this strategy precluded the opportunity to include patients who rescheduled and attended their GC appointment. Thus, recruitment procedures were revised so that patients who scheduled a new appointment were mailed an additional packet. Patients who failed to reschedule their appointment between the date of their canceled appointment and July 2010 (end of data collection) were considered ineligible. Participants received a $25.00 and $20.00 gift card for completing T1 and T2, respectively.
Measures
Participants had the option of completing paper or web-based surveys. Data on sociodemographic and clinical characteristics were collected at baseline; primary outcome data were collected at T1 and T2.
Sociodemographic and clinical characteristics
Sociodemographic and clinical characteristics obtained from patient questionnaires or medical records review included: age at diagnosis, current stage of BC (1, 2/3, 4, unstaged, other [e.g., unknown]), previous surgery (yes [i.e., breast conservation lumpectomy, unilateral mastectomy following biopsy, contralateral mastectomy following unilateral mastectomy, or bilateral mastectomy], no [have not had surgery]), and primary payor at diagnosis (private insurance, public insurance, no insurance, other). Additional data collected via self-report questionnaires included: education (high school or less; vocational school and some college; college graduate and beyond), total household income at time of diagnosis (< 35k, > 35-≤ 50K, > 50K), marital status (married, other), and race (Black, White, other).
HBOC knowledge
A 15-item, adapted version of the National Center for Human Genome Research (NCHGR) Knowledge Scale[34-37] was used to measure four aspects of HBOC genetics knowledge: (a) prevalence of BRCA gene mutations, (b) patterns of inheritance, (c) cancer risks associated with BRCA mutations, and (d) risk management options for women with a BRCA mutation. Items were scored as a 1 for correct and a 0 for incorrect or “don't know” responses, allowing for the calculation of an overall knowledge score ranging from 0-15. The scale had adequate internal consistency at baseline and following pretest GC, T1 α = .82 and T2 α = .81.
Cancer-related distress
The 15-item Impact of Events Scale (IES)[38] measure of current, subjective distress was used to assess the frequency of intrusive thoughts or avoidance related to a specific stressor. Intrusion is characterized by repetitive thoughts, mental images, disturbing dreams, and repetitive behavior. Avoidance is associated with denial of consequences from an event, blunting feelings, and emotional numbness related to an event. Respondents were asked to indicate how frequently (0 = not at all, 1 = rarely, 3 = sometimes, 5 = often) they experienced each indicator of distress during the past 7 days due to their BC diagnosis. Overall cancer-related distress scores range from 0–75, intrusion subscale scores range from 0–35, and avoidance subscale scores range from 0–40. The IES has been found to be a reliable and valid instrument for cancer-related distress among women at increased risk for hereditary BC[39, 41] and was recently used in a study of newly diagnosed BC patients undergoing GC and GT.[42] In the current study, Cronbach's alpha for the overall distress scale was .92 and .92 at T1 and T2, respectively; α = .88 and .91 for the intrusion subscale at T1 and T2, respectively, and α = .81 and .82 for the avoidance subscale at T1 and T2, respectively.
Decisional conflict
The 16-item Decisional Conflict Scale (DCS)[43, 44] scale was used to assess patients' decisional conflict over GT and includes five subscales: (a) perceptions of being informed (i.e., feeling informed about options, risks, and benefits; informed subscale), (b) degree of clarity regarding their personal values attached to the pros and cons of their decision (i.e., feeling clear about personal values attached to benefits and risks; values clarity subscale), (c) perception of decisional support (i.e., feeling supported by others in decision-making; support subscale), (d) degree of certainty about the particular health decision that is to be made (i.e., feeling clear about, certain of, and at ease with the best personal choice; uncertainty subscale), and (e) perceived effective decision making (i.e., perceptions about whether decisions are informed, are consistent with personal values, will be implemented, and are satisfactory; effective decision subscale). After subscale scores were summed and divided by the total number of items, 1 point was subtracted from each subscale's total. These scores were then multiplied by 25, resulting in final scores ranging from 0 (low decisional conflict) to 100 (high decisional conflict). This scale has been shown to be reliable and valid for individuals making a variety of health-related decisions.[43, 45-50] Cronbach's α for the overall decisional conflict scale in the current study was .94 and .99 for T1 and T2, respectively; alphas for the: (a) informed subscale were .90 and .97, (b) values clarity subscale were .91 and .98, (c) support subscale were .57 and .97, (d) uncertainty subscale were .85 and .97, and (e) effective decision subscale were .89 and .98, at T1 and T2, respectively.
Data Analysis
Descriptive statistics were summarized to characterize the total sample and each group. Scoring guidelines for each survey instrument were used to compute composite and subscale scores. Distributional characteristics of the composite and subscale variables for both patient groups were examined using histograms. Prior to conducting within-group analyses, Wilcoxon rank-sum tests were performed to assess differences between BDS and ADS patient groups: (a) at baseline, or pre-GC on HBOC knowledge, cancer-related distress, and decisional conflict (and all related subscales), and (b) on variables constructed to measure changes from pre- to post-GC reports on HBOC knowledge, cancer-related distress, and decisional conflict (and all related subscales). For each group, the changes from pre- to post-GC cancer-related knowledge, BC- related distress, and decisional conflict, including subscales where appropriate, were compared using Wilcoxon signed-rank tests. All tests were two-sided, with alpha set at the conventional level of 0.05. Statistical analyses were conducted using SAS (Version 9.2).
Results
A total of 223 patients were identified. Of these, 87 patients did not meet eligibility requirements (e.g., previous participation in GC and/or testing for HBOC, rescheduled GC appointment more than three times, non-English speaking). Of the remaining 136 eligible participants, 114 consented and completed the T1 survey, resulting in an 83.8% response rate. Of 114, there were 87 ADS patients (i.e., had undergone ≥ one surgery) and 16 BDS patients (i.e., had not undergone definitive surgery) at T1. Eleven patients did not know whether they had undergone previous surgery at T1; these patients were excluded from the current analyses given study aims, resulting in a total of 103 patients across groups at T1. Ninety-three patients completed the T2 assessment.
As shown in Table 1, participants were, on average, 49 years old (range = 24-69). The majority was married (68.9%), had previously undergone surgery (84.5%), and had private insurance at the time of diagnosis (52.4%). About 38% of participants were diagnosed in stage 2/3. Approximately 81% of participants were White, 49.5% had college or post-graduate degrees, and 37.9% had a household income > 50K prior to diagnosis.
Table 1. Sociodemographic and Clinical Characteristics of Study Participants.
| Total Sample (N = 103) | Before Definitive Surgery (BDS) Patients (n = 16) | After Definitive Surgery (ADS) Patients (n = 87) | p-value* | |
|---|---|---|---|---|
| Age at Diagnosis: Mean (SD) [Range] | 49.2 (10.7) [24-69] | 45.9 (12.5) [24-67] | 49.8 (10.2) [24-69] | .173 |
| n (%)a | ||||
| Current Stage | ||||
| Stage 1 | 26 (25.2) | 1 (6.3) | 25 (29.8) | .078 |
| Stage 2/3 | 39 (37.9) | 9 (56.3) | 30 (35.7) | |
| Stage 4 | 8 (7.8) | 3 (18.8) | 5 (6.0) | |
| Unstaged | 21 (20.4) | 3 (18.8) | 18 (21.4) | |
| Other (unknown; bilateral breast) | 6 (5.8) | 0 | 6 (7.1) | |
| Primary Payor at Diagnosis | ||||
| Private Insurance | 54 (52.4) | 9 (60) | 45 (54.2) | .136 |
| Public Insurance | 31 (30.1) | 2 (13.3) | 29 (34.9) | |
| No Insurance | 2 (1.9) | 1 (6.7) | 1 (1.2) | |
| Other | 11 (10.7) | 3 (20) | 8 (9.6) | |
| Education | ||||
| High School or Less | 15 (14.6) | 1 (6.3) | 14 (16.1) | .662 |
| Vocational School and Some College | 37 (35.9) | 6 (37.5) | 31 (35.6) | |
| College Graduate and Beyond | 51 (49.5) | 9 (56.3) | 42 (48.3) | |
| Total Household Income Prior to Diagnosis | ||||
| ≤ 35K | 28 (27.2) | 4 (28.6) | 24 (32.9) | .866 |
| > 35-≤ 50K | 20 (19.4) | 4 (28.6) | 16 (21.9) | |
| > 50K | 39 (37.9) | 6 (42.9) | 33 (45.2) | |
| Marital Status | ||||
| Marriedb | 71 (68.9) | 11 (68.8) | 60 (69.0) | 1.00 |
| Other | 32 (31.1) | 5 (31.3) | 27 (31.0) | |
| Race | ||||
| Black | 8 (7.8) | 1 (6.3) | 7 (8.1) | 1.00 |
| White | 83 (80.6) | 13 (81.3) | 70 (80.5) | |
| Other | 12 (11.7) | 2 (12.5) | 10 (11.5) | |
Percentages may not add up to 100 due to missing data.
Includes common law and living with domestic partner.
Wilcoxon signed-rank tests conducted to compare sociodemographic and clinical characteristics between groups.
There were no differences between groups at baseline or in changes from pre- to post-GC on HBOC knowledge, cancer-related distress, and decisional conflict (nor on any related subscales; data not shown).
As for within-group differences on study outcomes, both BDS and ADS patients showed significant increases in HBOC knowledge from pre- to post-GC (median change = 4.0, p = .004, and 3.0, p < .001, for BDS and ADS patients, respectively; see Table 2). With regard to psychological distress, results showed significant decreases in overall cancer-related distress (p = .041) and intrusive thoughts (p = .014) from pre- to post-GC for BDS patients. For ADS patients, there were non-significant downward trends for distress from baseline to post-GC (p = .303, p = .274, and p = .646 for median changes in overall distress, intrusion, and avoidance for ADS patients, respectively; see Figure 2).
Table 2. Changes in Cancer-Related Knowledge and Decisional Conflict Regarding GTa From Pre- to Post-GCb In BDSc and ADSd patients.
| Median [Range]e | p-value** | |||
|---|---|---|---|---|
| Pre-GC | Post-GC | Change (Post-Pre) | ||
| BDS Patients (n = 16) | ||||
| HBOC Knowledge | (n = 16) 7.0 [2-11] | (n = 10) 10.0 [7-15] | (n = 10) 4 0[-1, 8] | .004 |
| Overall Decisional Conflict | (n = 15) 23.4 [0.0, 60.9] | (n = 11) 25.0 [0.0, 100.0] | (n = 10) 3.1[-34, 89] | .748 |
| Uncertainty | (n = 15) 25.0 [0.0, 58.3] | (n = 11) 25.0 [0.0,100.0] | (n = 10) 0.0[-91.7, 33.3] | .625 |
| Informed | (n = 16) 29.2 [0.0, 91.7] | (n = 11) 25.0 [0.0,100.0] | (n = 11) 0.0[-41.7, 75.0] | .383 |
| Values Clarity | (n = 16) 25.0 [0.0, 58.3] | (n = 11) 25.0 [0.0,100.0] | (n = 11) 0.0[-42, 75] | .818 |
| Support | (n = 16) 25.0 [0.0, 50.0] | (n = 11) 16.7 [0.0,100.0] | (n = 11) 8.3[-33, 100] | .406 |
| Perceived Effective Decision Making | (n = 16) 25.0 [0.0, 56.3] | (n = 11) 25.0 [0.0,100.0] | (n = 11) 0.0[-50, 100] | .719 |
| ADS Patients (n = 87) | ||||
| HBOC Knowledge | (n = 85) 7.0 [0-14] | (n = 78) 10.0 [1-14] | (n = 76) 3.0[-10, 11] | <.001 |
| Overall Decisional Conflict | (n = 83) 29.7 [0.0, 68.8] | (n = 78) 18.0 [0.0, 100.0] | (n = 74) -10.2[-48, 100] | .056 |
| Uncertainty | (n = 87) 25.0 [0.0, 91.7] | (n = 80) 16.7 [0.0, 100.0] | (n = 80) 0.0[-100, 75] | .660 |
| Informed | (n = 85) 50.0 [0.0,100.0] | (n = 80) 25.0 [0.0, 100.0] | (n = 78) -25.0[-75, 100] | <.001 |
| Values Clarity | (n = 87) 33.3 [0.0, 100.0] | (n = 81) 25.0 [0.0, 100.0] | (n = 81) -8.3[-75, 100] | .075 |
| Support | (n = 86) 25.0 [0.0, 66.7] | (n = 80) 16.7 [0.0, 100.0] | (n = 79) -8.3[-50, 100] | .206 |
| Perceived Effective Decision Making | (n = 85) 25.0 [0.0, 62.5] | (n = 79) 12.5 [0.0, 100.0] | (n = 77) 0.0[-63, 100] | .989 |
Figure 2.
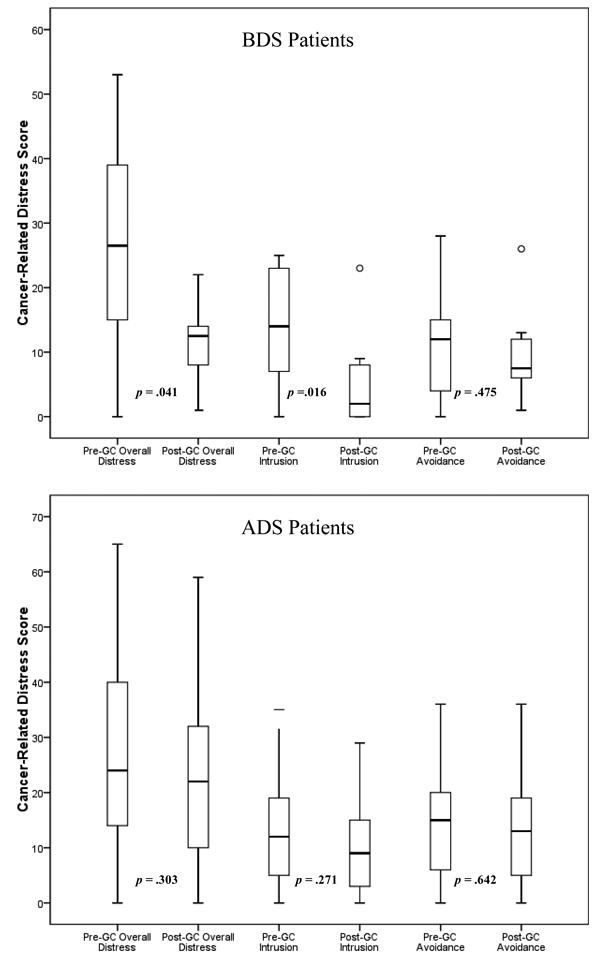
Changes in breast cancer-related distress (overall and by subscales) from pre-to post-GC in before definitive surgery (BDS; n = 16) patients (above panel) and after definitive surgery (ADS; n = 87) patients (below panel); Wilcoxon signed-rank tests conducted to compare median changes over time.
In terms of decisional conflict regarding GT, BDS patient reports on overall and subscales of decisional conflict trended towards increases in conflict with the exception of the informed subscale of decisional conflict which trended downward, however, changes were not statistically significant (see Table 3). ADS patients reported decreases in overall decisional conflict that approached marginal significance (median change = -10.2, p = .056, see Table 3) and significant decreases in the informed subscale (median change = -25.0, p < .001).
Table 3. Changes in Cancer-Related Knowledge and Decisional Conflict Regarding GTa From Pre- to Post-GCb In BDSc and ADSd patients.
| Median [Range]e | p-value** | |||
|---|---|---|---|---|
| Pre-GC | Post-GC | Change (Post-Pre) | ||
| BDS Patients (n = 16) | ||||
| HBOC Knowledge | (n = 16) 7.0 [2-11] | (n = 10) 10.0 [7-15] | (n = 10) 4 0[-1, 8] | .004 |
| Overall Decisional Conflict | (n = 15) 23.4 [0.0, 60.9] | (n = 11) 25.0 [0.0, 100.0] | (n = 10) 3.1[-34, 89] | .748 |
| Uncertainty | (n = 15) 25.0 [0.0, 58.3] | (n = 11) 25.0 [0.0,100.0] | (n = 10) 0.0[-91.7, 33.3] | .625 |
| Informed | (n = 16) 29.2 [0.0, 91.7] | (n = 11) 25.0 [0.0,100.0] | (n = 11) 0.0[-41.7, 75.0] | .383 |
| Values Clarity | (n = 16) 25.0 [0.0, 58.3] | (n = 11) 25.0 [0.0,100.0] | (n = 11) 0.0[-42, 75] | .818 |
| Support | (n = 16) 25.0 [0.0, 50.0] | (n = 11) 16.7 [0.0,100.0] | (n = 11) 8.3[-33, 100] | .406 |
| Perceived Effective Decision Making | (n = 16) 25.0 [0.0, 56.3] | (n = 11) 25.0 [0.0,100.0] | (n = 11) 0.0[-50, 100] | .719 |
| ADS Patients (n = 87) | ||||
| HBOC Knowledge | (n = 85) 7.0 [0-14] | (n = 78) 10.0 [1-14] | (n = 76) 3.0[-10, 11] | <.001 |
| Overall Decisional Conflict | (n = 83) 29.7 [0.0, 68.8] | (n = 78) 18.0 [0.0, 100.0] | (n = 74) -10.2[-48, 100] | .056 |
| Uncertainty | (n = 87) 25.0 [0.0, 91.7] | (n = 80) 16.7 [0.0, 100.0] | (n = 80) 0.0[-100, 75] | .660 |
| Informed | (n = 85) 50.0 [0.0,100.0] | (n = 80) 25.0 [0.0, 100.0] | (n = 78) -25.0[-75, 100] | <.001 |
| Values Clarity | (n = 87) 33.3 [0.0, 100.0] | (n = 81) 25.0 [0.0, 100.0] | (n = 81) -8.3[-75, 100] | .075 |
| Support | (n = 86) 25.0 [0.0, 66.7] | (n = 80) 16.7 [0.0, 100.0] | (n = 79) -8.3[-50, 100] | .206 |
| Perceived Effective Decision Making | (n = 85) 25.0 [0.0, 62.5] | (n = 79) 12.5 [0.0, 100.0] | (n = 77) 0.0[-63, 100] | .989 |
GT = Genetic testing.
GC = Genetic counseling.
BDS = Before definitive surgery.
ADS = After definitive surgery.
Scores for cancer-related knowledge range from 0 (no items correct) to 11 (all items correct; scores for decisional conflict range from 0 (no decisional conflict) to 100 (extremely high decisional conflict).
Wilcoxon signed-rank tests conducted to compare changes in outcomes, including subscales, from pre- to post-GC in BDS and ADS patients
Discussion
Several studies[51-54] have documented underutilization of GC and GT, particularly among newly diagnosed BC patients.[55] This may be due, in part, to patient lack of understanding of how the GC and GT process can inform their current treatment decisions[31, 32] and/or concerns regarding the psychological impact of this process at an already stressful time.[31] Similarly, healthcare providers may be concerned about a patient's ability to cope effectively with this process at a time when they are dealing with a new BC diagnosis.[33] However, no study to date has specifically explored whether BDS and ADS BC patients have similar responses to pretest GC. This pilot study assessed HBOC knowledge, cancer-related distress, and decisional conflict before and after pretest GC for BRCA mutations in high-risk BC patients who have and have not had definitive surgery. Although there were no differences between groups at baseline or in changes between T1 and T2 on study outcomes, there were several notable within-group changes.
Similar to the results of other studies assessing knowledge before and after pretest GC in women at increased risk of developing hereditary BC,[30] both patient groups (BDS and ADS patients) reported increases in HBOC knowledge after GC compared to baseline. These findings are encouraging, given increased patient knowledge is considered an important goal of pretest GC.[56]
With regard to psychological distress, BDS patients experienced significant decreases in cancer-related distress and in intrusive thoughts between T1 and T2. These findings support previous research demonstrating reductions in baseline cancer worry following participation in pretest GC.[57] In this sense, pretest GC may be psychologically protective for BDS patients in terms of cognitive intrusion, rather than distressing. Although levels of cognitive avoidance declined after GC compared to baseline for BDS patients, changes were not significant. Reduction in psychological distress in ADS patients demonstrated a downward trend, but also did not reach significance. These results are consistent with those reported by at risk, unaffected women[58] and by women who have undergone surgery.[30] Further research is needed to understand the impact of GC on cognitive avoidance.
Although not statistically significant, BDS patients reported increased levels of overall decisional conflict related to the decision to have GT. GC protocols may need to be adapted to meet specific needs and perspectives of BDS BC patients to ensure timely and effective decision making after pretest GC. For example, BC patients who decline BRCA GT may be more likely to report increased “cons” (rather than decreased “pros”) than those who choose to undergo GT.[54] Therefore, BDS patient-genetic counselor dialogue may need to focus on addressing perceived risks associated with GT and how those align with patient values and satisfaction.
Conversely, trends suggest pretest GC decreases overall decisional conflict for ADS patients, with significant decreases in conflict regarding informed decision making (e.g., knowing the pros and cons of each option and which is most salient personally). It is possible that ADS patients gain increased understanding of the benefits and risks of previous and potential treatment and surgical options, thereby alleviating decisional conflict over GT and promoting future informative treatment decisions.
Results of the current study, however, should be examined in light of its limitations. First, the small sample size for BDS patients yielded limited statistical power for our analyses, potentially leading to Type II errors. However, our smaller sample of BDS patients likely reflects the current clinical reality that fewer newly diagnosed BC patients are being referred for and participating in pretest GC before undergoing surgical treatment.[55] It is also important to consider the heterogeneity that may exist within our BDS group. It is possible that this group may include patients without an imminent surgical decision (e.g., women who may be undergoing neoadjuvant chemotherapy prior to surgical treatment). Upon reviewing medical records for the 16 BDS patients, clinic notes confirm that one of the 16 non-surgical patients had surgery prior to the T2 assessment. In addition, we assessed whether any of these patients underwent surgery prior to T3 (post-GT). Eight of the 16 patients classified as non-surgical at T1 and T2 had undergone surgery prior to the six-month follow-up at T3 (post-GT). This study also did not have a comparison group of BC patients who did not receive pretest GC. Previous research including both an ADS and comparison group, however, suggest there are no differences between groups in general anxiety, depression, or breast-cancer related distress.[30] In terms of ADS patients, 30% received GC at least more than one year since their diagnosis. Post-hoc analyses demonstrated that ADS patients who were diagnosed more than one year since GC experienced significantly different changes in distress and intrusion than those who were more recently diagnosed (data not shown). Examining effects of time since diagnosis on GC and GT outcomes, particularly within surgical patients, warrants future investigation.
Additionally, our study evaluated the impact of pre-test GC. However, it is very likely that discussions and informal counseling regarding hereditary cancer risk began prior to the actual pre-test GC session, namely with surgeons, medical oncologists, nurse navigators or other health care professionals that are involved in the care of BC patients. We conducted a post hoc frequency analysis to assess the proportion of BDS and ADS patients who previously discussed GC with a healthcare professional. Approximately 94% of BDS and 84% of ADS patients reported discussing GC with a healthcare professional prior to attending the GC session. A Fisher's exact test was conducted to determine whether there were significant differences between groups regarding prior discussion of GC. Results suggest no significant differences between BDS and ADS groups in terms of prior discussion of GC, p = .455.
All patients in the current study were afforded an in-person GC session by a master's prepared genetic counselor focusing in oncology, which may not reflect delivery of pretest GC across the US.[59] However, differences in delivery may dissipate over time based on 2012 American College of Surgeons' Commission on Cancer accreditation guidelines that specify risk assessment and GC offered by a qualified genetic professional as a clinical service to ensure patient-centered quality of care. Also, the majority of patients was non-Hispanic White; thus, findings from this study may not be generalizable to BC patients from other racial and ethnic populations. It is important to note as well that while BC patients in this study were referred for GC based on NCCN and study criteria, patients may differ in terms of the number and types of criterions met. For example, some women may have been referred for early-onset BC (under age 50) and strong family history, whereas others may have been referred based on family history. These differences may affect GC outcomes. Therefore, future investigations should attempt to parcel out these effects.
Although BDS and ADS patients did not exhibit increases in psychological distress in this study, BC patients who are younger, single with little social support, less optimistic, employ maladaptive coping, report lower quality of life, or exhibit high distress at baseline are more likely to experience adverse psychological effects.[30] Future studies with larger, nationally representative samples of BC patients should examine multiple types of psychological distress (e.g., anxiety, depression) and the potential moderating effects of age; marital status; and personality, coping, and quality of life variables in BDS and ADS patient outcomes.
Synopsis.
Longitudinal data were used to examine changes in cancer-related knowledge, distress, and decisional conflict from pretest- to post-genetic counseling (GC) in before (BDS) and after (ADS) definitive surgery breast cancer patients. Cancer knowledge increased for both patient groups, distress decreased in BDS, and informed decision making improved in ADS patients.
Acknowledgments
This research was conducted with funding from the American Cancer Society [MRSG CPPB-111062].The work contained within this publication was supported in part by the Survey Methods Core Facility at Moffitt Cancer Center.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest to disclose.
Contributor Information
Juliette Christie, Email: Juliette.Christie@moffitt.org, Health Outcomes and Behavior Program, Moffitt Cancer Center, 12902 Magnolia Drive MRC-CANCONT, Tampa, FL 33612, Phone: 813-745-4668, Fax: 813-745-6525.
Gwendolyn P. Quinn, Health Outcomes and Behavior Program, Moffitt Cancer Center, Department of Oncologic Science, College of Medicine, University of South Florida.
Teri Malo, Health Outcomes and Behavior Program, Moffitt Cancer Center.
Ji-Hyun Lee, Biostatistics, Moffitt Cancer Center, Department of Oncologic Science, College of Medicine, University of South Florida.
Xiuhua Zhao, Biostatistics, Moffitt Cancer Center.
Jessica McIntyre, Health Outcomes and Behavior Program, Moffitt Cancer Center.
Jennifer Brzosowicz, Lifetime Cancer Screening & Prevention Center, Moffitt Cancer Center.
Paul B. Jacobsen, Division of Population Science, Moffitt Cancer Center, Department of Oncologic Science, College of Medicine, University of South Florida, Aging Studies, College of Behavioral and Community Sciences, University of South Florida.
Susan T. Vadaparampil, Health Outcomes and Behavior Program, Moffitt Cancer Center, Department of Oncologic Science, College of Medicine, University of South Florida.
References
- 1.Graeser MK, Engel C, Rhiem K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27:5887–5892. doi: 10.1200/JCO.2008.19.9430. [DOI] [PubMed] [Google Scholar]
- 2.Malone KE, Begg CB, Haile RW, et al. Population-based study of the risk of second primary contralateral breast cancer associated with carrying a mutation in BRCA1 or BRCA2. J Clin Oncol. 28:2404–2410. doi: 10.1200/JCO.2009.24.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metcalfe KA, Lynch HT, Ghadirian P, et al. The risk of ovarian cancer after breast cancer in BRCA1 and BRCA2 carriers. Gynecol Oncol. 2005;96:222–226. doi: 10.1016/j.ygyno.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Thompson W, Semenciw R, Mao Y. Epidemiology of contralateral breast cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:855–861. [PubMed] [Google Scholar]
- 5.Brekelmans CT, Tilanus-Linthorst MM, Seynaeve C, et al. Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1- and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur J Cancer. 2007;43:867–876. doi: 10.1016/j.ejca.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Daly M, Axilbund JE, Bryant E, et al. The NCCN Genetic/Familial High-Risk Assessment: Breast and Ovarian Clinical Practice Guideline, version 1. [Accessed November 19th, 2006];2006 Available at http://nccn.org/physician_gls/indiex.html. To view most recent and complete version of guideline, to www.nccn.org. In Edition 2006.
- 7.Boughey JC, Hoskin TL, Degnim AC, et al. Contralateral prophylactic mastectomy is associated with a survival advantage in high-risk women with a personal history of breast cancer. Ann Surg Oncol. 17:2702–2709. doi: 10.1245/s10434-010-1136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finch A, Beiner M, Lubinski J, et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. JAMA. 2006;296:185–192. doi: 10.1001/jama.296.2.185. [DOI] [PubMed] [Google Scholar]
- 10.Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2004;22:1055–1062. doi: 10.1200/JCO.2004.04.188. [DOI] [PubMed] [Google Scholar]
- 11.Rebbeck TR, Levin AM, Eisen A, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst. 1999;91:1475–1479. doi: 10.1093/jnci/91.17.1475. [DOI] [PubMed] [Google Scholar]
- 12.Gronwald J, Tung N, Foulkes WD, et al. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer. 2006;118:2281–2284. doi: 10.1002/ijc.21536. [DOI] [PubMed] [Google Scholar]
- 13.King MC, Wieand S, Hale K, et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA. 2001;286:2251–2256. doi: 10.1001/jama.286.18.2251. [DOI] [PubMed] [Google Scholar]
- 14.Narod SA, Brunet JS, Ghadirian P, et al. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Hereditary Breast Cancer Clinical Study Group. Lancet. 2000;356:1876–1881. doi: 10.1016/s0140-6736(00)03258-x. [DOI] [PubMed] [Google Scholar]
- 15.Daly M, Axilbund JE, Bryant E, et al. The NCCN Genetic/Familial High-Risk Assessment: Breast and Ovarian Clinical Practice Guideline, version 1. 2009 Available at http://www.nccn.org/professionals/physician_gls/PDF/genetics_screening.pdf. To view most recent and complete version of guideline, to www.nccn.org. In Edition 2009.
- 16.Earle CC. Failing to plan is planning to fail: improving the quality of care with survivorship care plans. J Clin Oncol. 2006;24:5112–5116. doi: 10.1200/JCO.2006.06.5284. [DOI] [PubMed] [Google Scholar]
- 17.Hewitt M, Greenfield S, Stovall E. From cancer patient to cancer survivor: Lost in transition. Washington D.C: National Academies Press; 2005. [Google Scholar]
- 18.American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol. 2003;21:2397–2406. doi: 10.1200/JCO.2003.03.189. [DOI] [PubMed] [Google Scholar]
- 19.Robson ME, Storm CD, Weitzel J, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28:893–901. doi: 10.1200/JCO.2009.27.0660. [DOI] [PubMed] [Google Scholar]
- 20.Berliner JL, Fay AM. Risk assessment and genetic counseling for hereditary breast and ovarian cancer: recommendations of the National Society of Genetic Counselors. J Genet Couns. 2007;16:241–260. doi: 10.1007/s10897-007-9090-7. [DOI] [PubMed] [Google Scholar]
- 21.Fraser FC. Genetic counseling. Am J Hum Genet. 1974;26:636–661. [PMC free article] [PubMed] [Google Scholar]
- 22.Bernhardt BA, Biesecker BB, Mastromarino CL. Goals, benefits, and outcomes of genetic counseling: client and genetic counselor assessment. Am J Med Genet. 2000;94:189–197. doi: 10.1002/1096-8628(20000918)94:3<189::aid-ajmg3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Shiloh S, Avdor O, Goodman RM. Satisfaction with genetic counseling: dimensions and measurement. Am J Med Genet. 1990;37:522–529. doi: 10.1002/ajmg.1320370419. [DOI] [PubMed] [Google Scholar]
- 24.Pilnick A, Dingwall R. Research directions in genetic counselling: a review of the literature. Patient Educ Couns. 2001;44:95–105. doi: 10.1016/s0738-3991(00)00181-6. [DOI] [PubMed] [Google Scholar]
- 25.Braithwaite D, Emery J, Walter F, et al. Psychological impact of genetic counseling for familial cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2004;96:122–133. doi: 10.1093/jnci/djh017. [DOI] [PubMed] [Google Scholar]
- 26.Lerman C, Croyle RT, Tercyak KP, Hamann H. Genetic testing: psychological aspects and implications. J Consult Clin Psychol. 2002;70:784–797. doi: 10.1037//0022-006x.70.3.784. [DOI] [PubMed] [Google Scholar]
- 27.Meiser B. Psychological impact of genetic testing for cancer susceptibility: an update of the literature. Psychooncology. 2005;14:1060–1074. doi: 10.1002/pon.933. [DOI] [PubMed] [Google Scholar]
- 28.Hamann HA, Somers TJ, Smith AW, et al. Posttraumatic stress associated with cancer history and BRCA1/2 genetic testing. Psychosom Med. 2005;67:766–772. doi: 10.1097/01.psy.0000181273.74398.d7. [DOI] [PubMed] [Google Scholar]
- 29.Vadaparampil ST, Miree CA, Wilson C, Jacobsen PB. Psychosocial and Behavioral Impact of Genetic Counseling and Testing. Breast Dis. 2007;27:97–108. doi: 10.3233/bd-2007-27106. [DOI] [PubMed] [Google Scholar]
- 30.Schlich-Bakker KJ, Warlam-Rodenhuis CC, van Echtelt J, et al. Short term psychological distress in patients actively approached for genetic counselling after diagnosis of breast cancer. Eur J Cancer. 2006;42:2722–2728. doi: 10.1016/j.ejca.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 31.Vadaparampil ST, Quinn GP, Brzosowicz J, Miree CA. Experiences of genetic counseling for BRCA1/2 among recently diagnosed breast cancer patients: a qualitative inquiry. J Psychosoc Oncol. 2008;26:33–52. doi: 10.1080/07347330802359586. [DOI] [PubMed] [Google Scholar]
- 32.Geer KP, Ropka ME, Cohn WF, et al. Factors influencing patients' decisions to decline cancer genetic counseling services. J Genet Couns. 2001;10:25–40. doi: 10.1023/a:1009451213035. [DOI] [PubMed] [Google Scholar]
- 33.Nusbaum RH, Peshkin BN, DeMarco TA, Goodenberger M. BRCA 1/2 testing in patients with newly diagnosed breast cancer. Community Oncology. 2009;6:367–371. [Google Scholar]
- 34.Lerman C, Schwartz MD, Lin TH, et al. The influence of psychological distress on use of genetic testing for cancer risk. J Consult Clin Psychol. 1997;65:414–420. doi: 10.1037//0022-006x.65.3.414. [DOI] [PubMed] [Google Scholar]
- 35.Lerman C, Narod S, Schulman K, et al. BRCA1 testing in families with hereditary breast-ovarian cancer. A prospective study of patient decision making and outcomes. Jama. 1996;275:1885–1892. [PubMed] [Google Scholar]
- 36.Lerman C, Biesecker B, Benkendorf JL, et al. Controlled trial of pretest education approaches to enhance informed decision-making for BRCA1 gene testing. J Natl Cancer Inst. 1997;89:148–157. doi: 10.1093/jnci/89.2.148. [DOI] [PubMed] [Google Scholar]
- 37.Hopwood P, Shenton A, Lalloo F, et al. Risk perception and cancer worry: an exploratory study of the impact of genetic risk counselling in women with a family history of breast cancer. J Med Genet. 2001;38:139. doi: 10.1136/jmg.38.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Vadaparampil ST, Ropka ME, Stefanek ME. Measurement of Psychological Factors Associated with Genetic Testing for Hereditary Breast, Ovarian and Colon Cancers. Famililal Cancer. 2005 doi: 10.1007/s10689-004-1446-7. [DOI] [PubMed] [Google Scholar]
- 40.Wevers MR, Ausems MG, Verhoef S, et al. Behavioral and psychosocial effects of rapid genetic counseling and testing in newly diagnosed breast cancer patients: design of a multicenter randomized clinical trial. BMC Cancer. 2011;11:6. doi: 10.1186/1471-2407-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thewes B, Meiser B, Hickie IB. Psychometric properties of the Impact of Event Scale amongst women at increased risk for hereditary breast cancer. Psychooncology. 2001;10:459–468. doi: 10.1002/pon.533. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz MD, Lerman C, Brogan B, et al. Impact of BRCA1/BRCA2 counseling and testing on newly diagnosed breast cancer patients. J Clin Oncol. 2004;22:1823–1829. doi: 10.1200/JCO.2004.04.086. [DOI] [PubMed] [Google Scholar]
- 43.O'Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 44.o'Connor AM. Edition. Ottawa: Ottawa Hospital Research Institute; 1993. User Manual - Decisional Conflict Scale. updated 2010. [Google Scholar]
- 45.Cranney A, O'Connor AM, Jacobsen MJ, et al. Development and pilot testing of a decision aid for postmenopausal women with osteoporosis. Patient Educ Couns. 2002;47:245–255. doi: 10.1016/s0738-3991(01)00218-x. [DOI] [PubMed] [Google Scholar]
- 46.Man-Son-Hing M, Laupacis A, O'Connor AM, et al. A patient decision aid regarding antithrombotic therapy for stroke prevention in atrial fibrillation: a randomized controlled trial. Jama. 1999;282:737–743. doi: 10.1001/jama.282.8.737. [DOI] [PubMed] [Google Scholar]
- 47.Stacey D, DeGrasse C, Johnston L. Addressing the support needs of women at high risk for breast cancer: evidence- based care by advanced practice nurses. Oncol Nurs Forum. 2002;29:E77–84. doi: 10.1188/02.ONF.E77-E84. [DOI] [PubMed] [Google Scholar]
- 48.Sawka CA, Goel V, Mahut CA, et al. Development of a patient decision aid for choice of surgical treatment for breast cancer. Health Expect. 1998;1:23–36. doi: 10.1046/j.1369-6513.1998.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Connor AM, Tugwell P, Wells GA, et al. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Educ Couns. 1998;33:267–279. doi: 10.1016/s0738-3991(98)00026-3. [DOI] [PubMed] [Google Scholar]
- 50.O'Connor AM, Tugwell P, Wells GA, et al. Randomized trial of a portable, self-administered decision aid for postmenopausal women considering long-term preventive hormone therapy. Med Decis Making. 1998;18:295–303. doi: 10.1177/0272989X9801800307. [DOI] [PubMed] [Google Scholar]
- 51.Trivers KF, Baldwin LM, Miller JW, et al. Reported referral for genetic counseling or BRCA 1/2 testing among United States physicians: A Vignette-Based Study. Cancer. 2011 doi: 10.1002/cncr.26166. [DOI] [PubMed] [Google Scholar]
- 52.Meyer LA, Anderson ME, Lacour RA, et al. Evaluating women with ovarian cancer for BRCA1 and BRCA2 mutations: missed opportunities. Obstet Gynecol. 2010;115:945–952. doi: 10.1097/AOG.0b013e3181da08d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vadaparampil ST, Quinn GP, Miree CA, et al. Recall of and reactions to a surgeon referral letter for BRCA genetic counseling among high-risk breast cancer patients. Ann Surg Oncol. 2009;16:1973–1981. doi: 10.1245/s10434-009-0479-4. [DOI] [PubMed] [Google Scholar]
- 54.O'Neill SM, Peters JA, Vogel VG, et al. Referral to cancer genetic counseling: are there stages of readiness? Am J Med Genet C Semin Med Genet. 2006;142C:221–231. doi: 10.1002/ajmg.c.30109. [DOI] [PubMed] [Google Scholar]
- 55.Levy DE, Byfield SD, Comstock CB, et al. Underutilization of BRCA1/2 testing to guide breast cancer treatment: Black and Hispanic women particularly at risk. Genet Med. 2011 doi: 10.1097/GIM.0b013e3182091ba4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clarke A, Parsons E, Williams A. Outcomes and process in genetic counselling. Clin Genet. 1996;50:462–469. doi: 10.1111/j.1399-0004.1996.tb02713.x. [DOI] [PubMed] [Google Scholar]
- 57.Bowen DJ, Burke W, McTiernan A, et al. Breast cancer risk counseling improves women's functioning. Patient Educ Couns. 2004;53:79–86. doi: 10.1016/S0738-3991(03)00122-8. [DOI] [PubMed] [Google Scholar]
- 58.Meiser B, Halliday JL. What is the impact of genetic counselling in women at increased risk of developing hereditary breast cancer? A meta-analytic review. Soc Sci Med. 2002;54:1463–1470. doi: 10.1016/s0277-9536(01)00133-2. [DOI] [PubMed] [Google Scholar]
- 59.Bellcross CA, Kolor K, Goddard KA, et al. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 2011;40:61–66. doi: 10.1016/j.amepre.2010.09.027. [DOI] [PubMed] [Google Scholar]


