Figure 1. Schematic and in vitro characterizations.
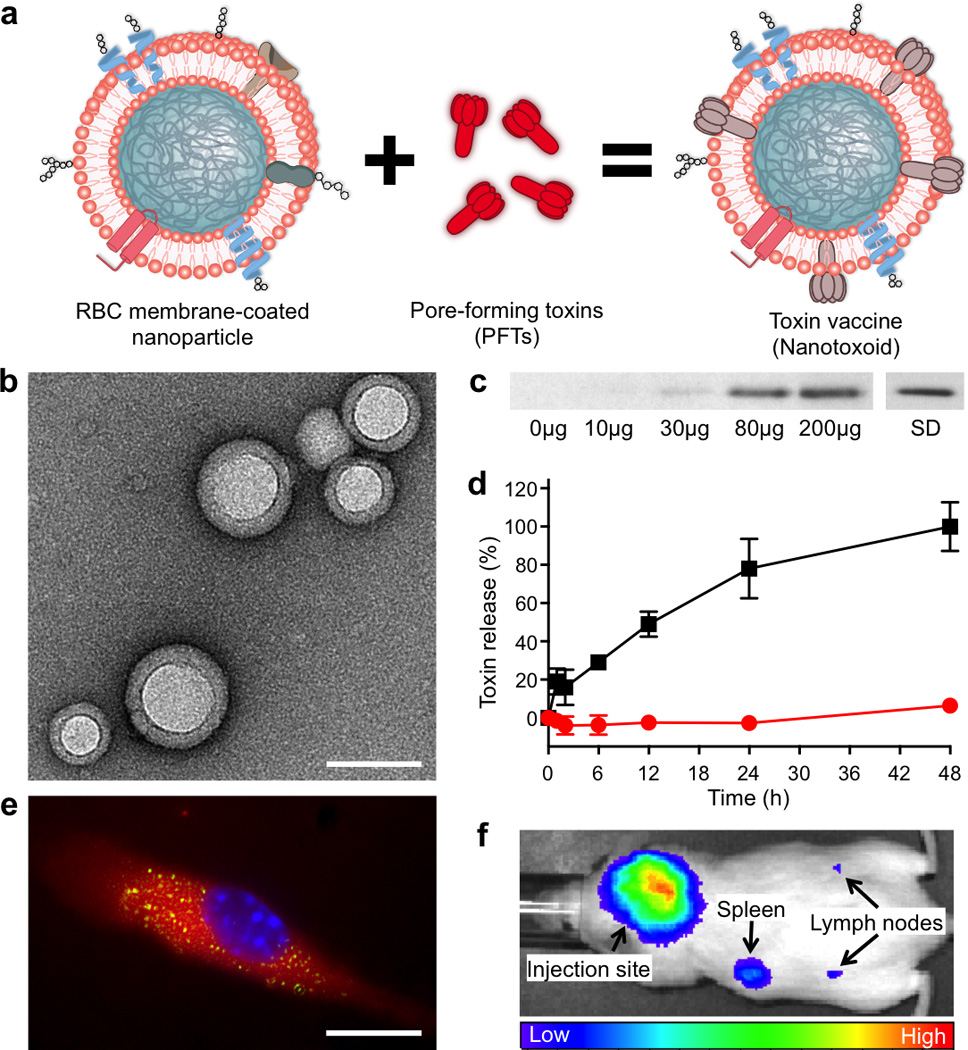
(a) Schematic preparation of nanoparticle-detained toxins, denoted as nanotoxoid, consisting of substrate-supported RBC membranes into which pore-forming toxins (PFTs) can spontaneously incorporate. (b) TEM visualization of the particle vectors with uranyl acetate staining (scale bar = 80 nm). (c) Western blotting results to verify the retention of 3 µg of staphylococcal α-hemolysin (Hla) by varying amounts of the particle vectors using 3 µg of free Hla as a standard (SD). (d) Release of toxin from the Hla-loaded nanotoxoids, denoted as nanotoxoid(Hla), over time in PBS buffer. Red circles indicate nanotoxoid(Hla) and black squares indicate free Hla. Error bars represent standard deviations of the mean. (e) Uptake of nanotoxoid(Hla) by a mouse dendritic cell (scale bar = 10 µm). The cell is membrane stained with DMPE-rhodamine B (red) and nuclei stained with DAPI (blue). FITC-labelled Hla (green) was used to monitor the toxin uptake. (f) Live, whole-body fluorescent imaging of nanotoxoid(Hla) at 1 h after subcutaneous administration.
