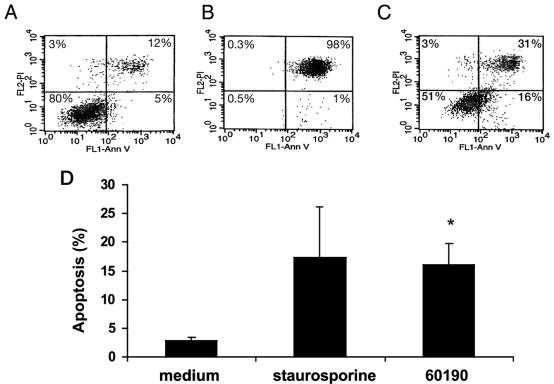
FIG. 4.
H. pylori induces apoptosis, as measured by annexin-V binding to externalized phosphatidylserine. RAW 264.7 cells were infected with H. pylori at an MOI of 50:1 for 24 h. Viable cells (annexin-V− PI−); nonviable, including late apoptotic or necrotic cells (annexin-V+ PI+ or annexin-V− PI+); and apoptotic cells (annexin-V+ PI−) were detected by the binding of Ann-V to externalized phospatidylserine in conjunction with PI, a dye excluded from viable cells. (A, B, and C) One FACS analysis representative of three individual experiments. (A) Eighty percent of uninfected RAW 264.7 cells were viable (annexin-V− PI−), while only 5% were apoptotic (annexin-V+ PI−). (B) One percent of RAW 264.7 cells treated with 1 μM staurosporine (positive control for apoptosis) were apoptotic (annexin-V+ PI−), and 98% of RAW 264.7 cells were nonviable (annexin-V+ PI+). (C) Sixteen percent of RAW 264.7 cells infected with H. pylori (60190) at an MOI of 50:1 for 24 h were apoptotic (annexin-V+ PI−), and 31% of the cells were nonviable (annexin-V+ PI+). FL1, flow cytometry channel 1 to detect PI stain; FL2, flow cytometry channel 2 to detect annexin-V. (D) Combined results of three separate FACS analyses depicting the mean levels of apoptotic cells (annexin-V+ PI−). Staurosporine-treated cells showed an increase in apoptosis over uninfected controls. RAW 264.7 cells infected with H. pylori (60190) at an MOI of 50:1 for 24 h showed an increase in apoptosis in comparison with uninfected controls (16.0% ± 3.7% versus 2.8% ± 0.6%; Tukey-Kramer test; *, P < 0.05).