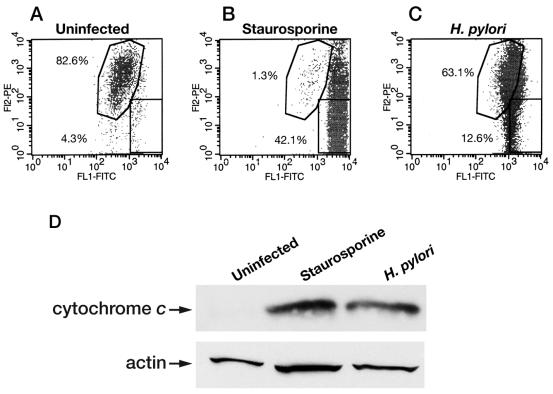
FIG. 8.
H. pylori infection leads to increased mitochondrial-membrane permeability and increased cytosolic cytochrome c in RAW 264.7 cells. Macrophages were harvested after H. pylori (60190) infection (MOI, 100:1; 24 h), and mitochondrial-membrane permeability was assessed using FACS analysis. (A, B, and C) One representative FACS analysis from three separate experiments is shown, where the x axis represents fluorescence levels of monomeric dye remaining in the cytosol of cells with increased mitochondrial-membrane permeability (green emission). In contrast, the y axis represents the fluorescence levels of the aggregated dye taken up and retained in the mitochondria of cells with intact mitochondrial membranes (red emission). (A) A total of 4.3% of uninfected RAW 264.7 cells show increased mitochondrial-membrane permeability, while 82.6% show intact mitochondrial-membrane permeability. (B) A total of 42.1% of staurosporine-treated macrophages have increased mitochondrial-membrane permeability, while only 1.3% display intact mitochondrial-membrane permeability. (C) At 24 h postinfection, 12.6% of H. pylori-infected RAW 264.7 cells display increased mitochondrial-membrane permeability. FL1, flow cytometry channel 1 to detect FITC; FL2, flow cytometry channel 2 to detect PE. (D) Infection with H. pylori leads to an increase in cytosolic cytochrome c protein levels, as assessed by Western blot analysis. In comparison with cytosolic extracts from uninfected RAW 264.7 cells, H. pylori strain 60190-infected RAW 264.7 cells (H. pylori) (MOI, 100:1; 24 h) show increased protein levels of cytosolic cytochrome c, similar to staurosporine-treated cells (Staurosporine) (1 μM; 24 h; positive control; n = 3).