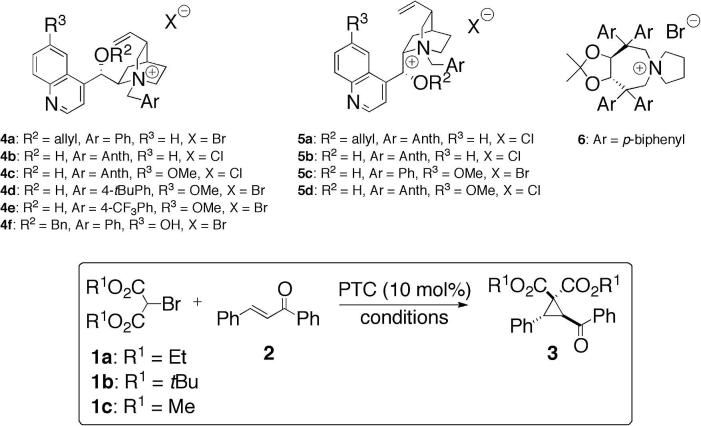
Table 1.
| Entry | 1 | Cat. | Solv. | Base (equiv) | Concd. | Yielda (%) | er b (+/−) |
|---|---|---|---|---|---|---|---|
| 1 | 1a | 4a | CH2Cl2 | Cs2CO3 (4×) | Ac | 70 | 51:49 |
| 2 | THF | 77 | 43:57 | ||||
| 3 | Toluene | 30 | 44:56 | ||||
| 4 | 4b | THF | 45 | 46:54 | |||
| 5 | 4c | 40 | 44:56 | ||||
| 6 | 5a | 41 | 51:49 | ||||
| 7 | 5c | 14 | 52:48 | ||||
| 8 | 5d | 55 | 57:43 | ||||
| 9 | 4c | Toluene | K3PO4 (10×) | 45 | 31:69 | ||
| 10 | 4a | K3PO4 (50%) (10×) | 84 | 50:50 | |||
| 11 | 4b | 36 | 38:62 | ||||
| 12 | 4c | 48 | 26:74 | ||||
| 13 | 4d | 57 | 40:60 | ||||
| 14 | 4e | 44 | 31:69 | ||||
| 15 | 4f | 67 | 50:50 | ||||
| 16 | 5b | 30 | 70:30 | ||||
| 17 | 5d | 45 | 73:27 | ||||
| 18 | 6 | 57 | 47:53 | ||||
| 19 | 4c | Mesitylene | 43 | 24:76 | |||
| 20 | Toluene | Li2CO3 (50%) (10×) | 7 | 21:79 | |||
| 21 | K2CO3 (50%) (10×) | 35 | 24:76 | ||||
| 22 | Mesitylene | 54 | 22:78 | ||||
| 23 | 1b | n.r. | — | ||||
| 24 | 1c | 63 | 17:83 | ||||
| 25 | Bc | 10 | 17:83 | ||||
| 26 | Cc | 54 | 15:85 | ||||
| 27 | Dc | 83 | 15:85 | ||||
| 28 | Ec | 82 | 13:87 | ||||
| 29 | Fc | 39 | 10:90 | ||||
| 30 | 5d | Ec | 61 | 75:25 |
Isolated yield.
Determined by HPLC using a chiral stationary phase.
A: 3 equiv 2, rt, 22 h, 0.15 M; B: 3 equiv 1a, RT, 22 h, 0.15 M; C: 3 equiv 2, rt, 46 h, 0.075 M; D: 6 equiv 2, rt, 46 h, 0.075 M; E: 6 equiv 2, 0 °C, 46 h, 0.075 M; F: 6 equiv 2, −20 °C, 46 h, 0.075 M.